Clinical features, diagnosis, and survival analysis of dogs with glioma
- PMID: 34117807
- PMCID: PMC8295679
- DOI: 10.1111/jvim.16199
Clinical features, diagnosis, and survival analysis of dogs with glioma
Abstract
Background: Gliomas in dogs remain poorly understood.
Objectives: To characterize the clinicopathologic findings, diagnostic imaging features and survival of a large sample of dogs with glioma using the Comparative Brain Tumor Consortium diagnostic classification.
Animals: Ninety-one dogs with histopathological diagnosis of glioma.
Methods: Multicentric retrospective case series. Signalment, clinicopathologic findings, diagnostic imaging characteristics, treatment, and outcome were used. Tumors were reclassified according to the new canine glioma diagnostic scheme.
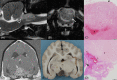
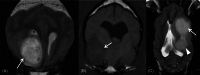
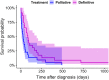
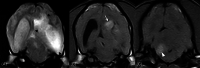
Results: No associations were found between clinicopathologic findings or survival and tumor type or grade. However, definitive treatments provided significantly (P = .03) improved median survival time (84 days; 95% confidence interval [CI], 45-190) compared to palliative treatment (26 days; 95% CI, 11-54). On magnetic resonance imaging (MRI), oligodendrogliomas were associated with smooth margins and T1-weighted hypointensity compared to astrocytomas (odds ratio [OR], 42.5; 95% CI, 2.42-744.97; P = .04; OR, 45.5; 95% CI, 5.78-333.33; P < .001, respectively) and undefined gliomas (OR, 84; 95% CI, 3.43-999.99; P = .02; OR, 32.3; 95% CI, 2.51-500.00; P = .008, respectively) and were more commonly in contact with the ventricles than astrocytomas (OR, 7.47; 95% CI, 1.03-53.95; P = .049). Tumor spread to neighboring brain structures was associated with high-grade glioma (OR, 6.02; 95% CI, 1.06-34.48; P = .04).
Conclusions and clinical importance: Dogs with gliomas have poor outcomes, but risk factors identified in survival analysis inform prognosis and the newly identified MRI characteristics could refine diagnosis of tumor type and grade.
Keywords: astrocytoma; dog; magnetic resonance imaging; oligodendroglioma; prognosis; tumor grade; undefined glioma.
© 2021 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures


Comment in
-
Letter regarding "Clinical features, diagnosis, and survival analysis of dogs with glioma".J Vet Intern Med. 2022 Sep;36(5):1566-1567. doi: 10.1111/jvim.16492. Epub 2022 Jul 27. J Vet Intern Med. 2022. PMID: 35894436 Free PMC article. No abstract available.
References
-
- McGrath JT. Intracranial pathology in the dog. Acta Neuropathol. 1962;1 (Suppl I):3‐4.
-
- Dorn CR, Taylor DO, Frye FL, et al. Survey of animal neoplasms in Alameda and Contra Costa counties, California. Methodology and description of cases. J Natl Cancer Inst. 1968;40:295‐305. - PubMed
-
- Song RB, Vite CH, Bradle CW, et al. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age and body weight. J Vet Intern Med. 2013;27:1143‐1152. - PubMed
-
- Bagley RS, Gavin PR, Moore MP, et al. Clinical signs associated with brain tumors in dogs: 97 cases (1992‐1997). J Am Vet Med Assoc. 1999;215:818‐819. - PubMed
-
- Snyder JM, Shofer FS, Van Winkle TJ, et al. Canine intracranial primary neoplasia: 173 cases (1986‐2003). J Vet Intern Med. 2008;22:172‐177. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

