Impact of superparamagnetic iron oxide nanoparticles on in vitro and in vivo radiosensitisation of cancer cells
- PMID: 34118963
- PMCID: PMC8199842
- DOI: 10.1186/s13014-021-01829-y
Impact of superparamagnetic iron oxide nanoparticles on in vitro and in vivo radiosensitisation of cancer cells
Abstract
Purpose: The recent implementation of MR-Linacs has highlighted theranostic opportunities of contrast agents in both imaging and radiotherapy. There is a lack of data exploring the potential of superparamagnetic iron oxide nanoparticles (SPIONs) as radiosensitisers. Through preclinical 225 kVp exposures, this study aimed to characterise the uptake and radiobiological effects of SPIONs in tumour cell models in vitro and to provide proof-of-principle application in a xenograft tumour model.
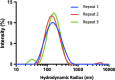
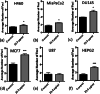
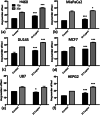
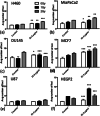
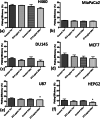
Methods: SPIONs were also characterised to determine their hydrodynamic radius using dynamic light scattering and uptake was measured using ICP-MS in 6 cancer cell lines; H460, MiaPaCa2, DU145, MCF7, U87 and HEPG2. The impact of SPIONs on radiobiological response was determined by measuring DNA damage using 53BP1 immunofluorescence and cell survival. Sensitisation Enhancement Ratios (SERs) were compared with the predicted Dose Enhancement Ratios (DEFs) based on physical absorption estimations. In vivo efficacy was demonstrated using a subcutaneous H460 xenograft tumour model in SCID mice by following intra-tumoural injection of SPIONs.
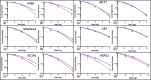
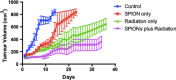
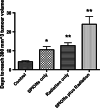
Results: The hydrodynamic radius was found to be between 110 and 130 nm, with evidence of being monodisperse in nature. SPIONs significantly increased DNA damage in all cell lines with the exception of U87 cells at a dose of 1 Gy, 1 h post-irradiation. Levels of DNA damage correlated with the cell survival, in which all cell lines except U87 cells showed an increased sensitivity (P < 0.05) in the linear quadratic curve fit for 1 h exposure to 23.5 μg/ml SPIONs. There was also a 30.1% increase in the number of DNA damage foci found for HEPG2 cells at 2 Gy. No strong correlation was found between SPION uptake and DNA damage at any dose, yet the biological consequences of SPIONs on radiosensitisation were found to be much greater, with SERs up to 1.28 ± 0.03, compared with predicted physical dose enhancement levels of 1.0001. In vivo, intra-tumoural injection of SPIONs combined with radiation showed significant tumour growth delay compared to animals treated with radiation or SPIONs alone (P < 0.05).
Conclusions: SPIONs showed radiosensitising effects in 5 out of 6 cancer cell lines. No correlation was found between the cell-specific uptake of SPIONs into the cells and DNA damage levels. The in vivo study found a significant decrease in the tumour growth rate.
Keywords: Nanoparticles; Radiobiology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

