Products for Monitoring Glucose Levels in the Human Body With Noninvasive Optical, Noninvasive Fluid Sampling, or Minimally Invasive Technologies
- PMID: 34120487
- PMCID: PMC8721558
- DOI: 10.1177/19322968211007212
Products for Monitoring Glucose Levels in the Human Body With Noninvasive Optical, Noninvasive Fluid Sampling, or Minimally Invasive Technologies
Abstract
Background: Conventional home blood glucose measurements require a sample of blood that is obtained by puncturing the skin at the fingertip. To avoid the pain associated with this procedure, there is high demand for medical products that allow glucose monitoring without blood sampling. In this review article, all such products are presented.
Methods: In order to identify such products, four different sources were used: (1) PubMed, (2) Google Patents, (3) Diabetes Technology Meeting Startup Showcase participants, and (4) experts in the field of glucose monitoring. The information obtained were filtered by using two inclusion criteria: (1) regulatory clearance, and/or (2) significant coverage in Google News starting in the year 2016, unless the article indicated that the product had been discontinued. The identified bloodless monitoring products were classified into three categories: (1) noninvasive optical, (2) noninvasive fluid sampling, and (3) minimally invasive devices.
Results: In total, 28 noninvasive optical, 6 noninvasive fluid sampling, and 31 minimally invasive glucose monitoring products were identified. Subsequently, these products were characterized according to their regulatory, technological, and consumer features. Products with regulatory clearance are described in greater detail according to their advantages and disadvantages, and with design images.
Conclusions: Based on favorable technological features, consumer features, and other advantages, several bloodless products are commercially available and promise to enhance diabetes management. Paths for future products are discussed with an emphasis on understanding existing barriers related to both technical and non-technical issues.
Keywords: fluid sampling; glucose; invasive; minimally invasive; noninvasive; optical.
Conflict of interest statement
Figures

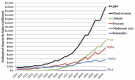

References
-
- American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S85-S99. https://care.diabetesjournals.org/content/44/Supplement_1/S85 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

