Role and Mechanism of Vitamin A Metabolism in the Pathophysiology of Parkinson's Disease
- PMID: 34120916
- PMCID: PMC8461657
- DOI: 10.3233/JPD-212671
Role and Mechanism of Vitamin A Metabolism in the Pathophysiology of Parkinson's Disease
Abstract
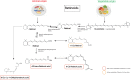
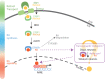
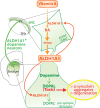
Evidence shows that altered retinoic acid signaling may contribute to the pathogenesis and pathophysiology of Parkinson's disease (PD). Retinoic acid is the bioactive derivative of the lipophilic vitamin A. Vitamin A is involved in several important homeostatic processes, such as cell differentiation, antioxidant activity, inflammation and neuronal plasticity. The role of vitamin A and its derivatives in the pathogenesis and pathophysiology of neurodegenerative diseases, and their potential as therapeutics, has drawn attention for more than 10 years. However, the literature sits in disparate fields. Vitamin A could act at the crossroad of multiple environmental and genetic factors of PD. The purpose of this review is to outline what is known about the role of vitamin A metabolism in the pathogenesis and pathophysiology of PD. We examine key biological systems and mechanisms that are under the control of vitamin A and its derivatives, which are (or could be) exploited for therapeutic potential in PD: the survival of dopaminergic neurons, oxidative stress, neuroinflammation, circadian rhythms, homeostasis of the enteric nervous system, and hormonal systems. We focus on the pivotal role of ALDH1A1, an enzyme expressed by dopaminergic neurons for the detoxification of these neurons, which is under the control of retinoic acid. By providing an integrated summary, this review will guide future studies on the potential role of vitamin A in the management of symptoms, health and wellbeing for PD patients.
Keywords: ALDH1A1; Neuroinflammation; RAR RXR receptors; oxidative stress; retinoic acid; vitamin A.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures

References
-
- Maden M (2007) Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8, 755–765. - PubMed
-
- Goodman AB (2006) Retinoid receptors, transporters, and metabolizers as therapeutic targets in late onset Alzheimer disease. J Cell Physiol 209, 598–603. - PubMed
-
- Lane MA, Bailey SJ (2005) Role of retinoid signalling in the adult brain. Prog Neurobiol 75, 275–293. - PubMed
-
- Shearer KD, Stoney PN, Morgan PJ, McCaffery PJ (2012) A vitamin for the brain. Trends Neurosci 35, 733–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

