A retrospective observational study to evaluate the clinical outcomes and routine management of patients with chronic lymphocytic leukaemia treated with idelalisib and rituximab in the UK and Ireland (RETRO-idel)
- PMID: 34121184
- PMCID: PMC8361941
- DOI: 10.1111/bjh.17475
A retrospective observational study to evaluate the clinical outcomes and routine management of patients with chronic lymphocytic leukaemia treated with idelalisib and rituximab in the UK and Ireland (RETRO-idel)
Abstract
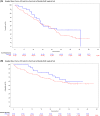
Idelalisib (IDL) is an oral first-in-class phosphatidylinositol 3-kinase delta (PI3Kδ) inhibitor approved for chronic lymphocytic leukaemia (CLL) alongside rituximab (R) since 2014. However, little data exist on routine practice. The RETRO-idel was a protocol-led, retrospective study of 110 patients [n = 27 front-line (1L)] who received IDL-R. The primary end-point was clinical overall response rate (ORR). The median (range) follow-up of the whole cohort was 30·2 (0·1-51·9) months. The median (range) age was 72 (48-89) years. Tumour protein p53-disruption was common [100% 1L, 32·5% relapsed/refractory (R/R)]. The best ORR (intention-to-treat) was 88·2% (1L 96·3%, R/R 85·5%). Overall, the median event-free survival (mEFS) was 20·3 months and time-to-next treatment was 29·2 months. The mEFS for 1L patients was 18·7 months and R/R patients was 21·7 months. The 3-year overall survival was 56·1% (95% confidence interval 45·7-65·3). IDL was discontinued in 87·3% (n = 96). More patients discontinued due to adverse events in the front-line setting (1L 63·0% vs. R/R 44·6%) and due to progressive disease in R/R patients (20·5% vs. 3·7% in 1L). Lower respiratory tract infection/pneumonia were reported in 34·5% (Grade ≥3, 19·1%), diarrhoea in 30·9% (Grade ≥3, 6·4%), and colitis in 9·1% (Grade ≥3, 5·5%). Overall, these data describe clear efficacy for IDL-R in routine practice. No new safety signals were identified, although careful management of known toxicities is required.
Keywords: B-cell receptor inhibitor; PI3K; chronic lymphocytic leukaemia; idelalisib; retrospective.
© 2021 Gilead Sciences Ltd. British Journal of Haematology published by John Wiley & Sons Ltd and British Society for Haematology.
Conflict of interest statement
Toby A. Eyre:
Figures
Comment in
-
Searching for a home: phosphoinositide 3-kinase inhibitors for chronic lymphocytic leukaemia in modern clinical practice.Br J Haematol. 2021 Jul;194(1):9-10. doi: 10.1111/bjh.17472. Epub 2021 Jun 15. Br J Haematol. 2021. PMID: 34128228 No abstract available.
References
-
- Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax‐rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous