Preinfection laboratory parameters may predict COVID-19 severity in tumor patients
- PMID: 34121360
- PMCID: PMC8267142
- DOI: 10.1002/cam4.4023
Preinfection laboratory parameters may predict COVID-19 severity in tumor patients
Abstract
Background: Infection with SARS-CoV-2 leads to COVID-19, the course of which is highly variable and depends on numerous patient-specific risk factors. Patients with tumor diseases are considered to be more susceptible to severe COVID-19; however, they also represent a heterogeneous group of individuals with variable risk. Identifying specific risk factors for a severe course of COVID-19 in patients with cancer is of great importance.
Methods: Patients diagnosed with solid tumors or hematological malignancies and PCR-confirmed SARS-CoV-2 infection were included into the multicentric ADHOK (Arbeitsgemeinschaft der Hämatologen und Onkologen im Krankenhaus e.V.) coronavirus tumor registry. Detailed information about the patients' cancer disease, treatment, and laboratory parameters prior to infection, was collected retrospectively. The outcome of the SARS-CoV-2 infection was graded according to the WHO.
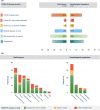
Results: A total of 195 patients (68% with solid neoplasms and 32% with hematological malignancies) were included in the registry. Overall, the course of the SARS-CoV-2 infection varied greatly, as 69% of all patients were either asymptomatic or encountered a mild to moderate course, while 23% of the cohort died from COVID-19. In multivariable analysis, preinfection laboratory parameters (determined at least 10 days and a median of 21 days before the first documentation of SARS-CoV-2 infection) significantly correlated with severe course of the disease. Out of these, the absolute neutrophil count prior to infection showed the strongest association with COVID-19-related death.
Conclusion: The course of COVID-19 in patients with tumor diseases is highly variable. Preinfection laboratory parameters may aid to identify patients at risk for severe COVID-19 at an early stage prior to infection with the virus. German Clinical Trials Register identification: DRKS00023012.
Keywords: COVID-19; SARS-CoV-2; biomarkers; cancer; neutrophils; tumor.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
There is no conflict of interest to disclose for any of the authors.
Figures


References
-
- Johns Hopkins Coronavirus Resource Center (CRC) . COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Accessed January 25, 2021. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

