Skeletal myopathy in CKD: a comparison of adenine-induced nephropathy and 5/6 nephrectomy models in mice
- PMID: 34121452
- PMCID: PMC8321803
- DOI: 10.1152/ajprenal.00117.2021
Skeletal myopathy in CKD: a comparison of adenine-induced nephropathy and 5/6 nephrectomy models in mice
Abstract
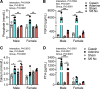
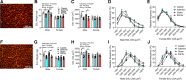
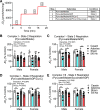
Preclinical animal models of chronic kidney disease (CKD) are critical to investigate the underlying mechanisms of disease and to evaluate the efficacy of novel therapeutics aimed to treat CKD-associated pathologies. The objective of the present study was to compare the adenine diet and 5/6 nephrectomy (Nx) CKD models in mice. Male and female 10-wk-old C57BL/6J mice (n = 5-9 mice/sex/group) were randomly allocated to CKD groups (0.2-0.15% adenine-supplemented diet or 5/6 Nx surgery) or the corresponding control groups (casein diet or sham surgery). Following the induction of CKD, the glomerular filtration rate was reduced to a similar level in both adenine and 5/6 Nx mice (adenine diet-fed male mice: 81.1 ± 41.9 µL/min vs. 5/6 Nx male mice: 160 ± 80.9 µL/min, P = 0.5875; adenine diet-fed female mice: 112.9 ± 32.4 µL/min vs. 5/6 Nx female mice: 107.0 ± 45.7 µL/min, P = 0.9995). Serum metabolomics analysis indicated that established uremic toxins were robustly elevated in both CKD models, although some differences were observed between CKD models (i.e., p-cresol sulfate). Dysregulated phosphate homeostasis was observed in the adenine model only, whereas Ca2+ homeostasis was disturbed in male mice with both CKD models. Compared with control mice, muscle mass and myofiber cross-sectional areas of the extensor digitorum longus and soleus muscles were ∼18-24% smaller in male CKD mice regardless of the model but were not different in female CKD mice (P > 0.05). Skeletal muscle mitochondrial respiratory function was significantly decreased (19-24%) in CKD mice in both models and sexes. These findings demonstrate that adenine diet and 5/6 Nx models of CKD have similar levels of renal dysfunction and skeletal myopathy. However, the adenine diet model demonstrated superior performance with regard to mortality (∼20-50% mortality for 5/6 Nx vs. 0% mortality for the adenine diet, P < 0.05 for both sexes) compared with the 5/6 Nx surgical model.NEW & NOTEWORTHY Numerous preclinical models of chronic kidney disease have been used to evaluate skeletal muscle pathology; however, direct comparisons of popular models are not available. In this study, we compared adenine-induced nephropathy and 5/6 nephrectomy models. Both models produced equivalent levels of muscle atrophy and mitochondrial impairment, but the adenine model exhibited lower mortality rates, higher consistency in uremic toxin levels, and dysregulated phosphate homeostasis compared with the 5/6 nephrectomy model.
Keywords: atrophy; cachexia; mitochondria; renal; uremia.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Gracia-Iguacel C, Gonzalez-Parra E, Perez-Gomez MV, Mahillo I, Egido J, Ortiz A, Carrero JJ. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia 33: 495–505, 2013. doi:10.3265/Nefrologia.pre2013.Apr.11979. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

