Metabolomic Characterization of Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS)
- PMID: 34121984
- PMCID: PMC8194687
- DOI: 10.3389/fnins.2021.645267
Metabolomic Characterization of Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS)
Abstract
Introduction: PANS is a controversial clinical entity, consisting of a complex constellation of psychiatric symptoms, adventitious changes, and expression of various serological alterations, likely sustained by an autoimmune/inflammatory disease. Detection of novel biomarkers of PANS is highly desirable for both diagnostic and therapeutic management of affected patients. Analysis of metabolites has proven useful in detecting biomarkers for other neuroimmune-psychiatric diseases. Here, we utilize the metabolomics approach to determine whether it is possible to define a specific metabolic pattern in patients affected by PANS compared to healthy subjects.
Design: This observational case-control study tested consecutive patients referred for PANS between June 2019 to May 2020. A PANS diagnosis was confirmed according to the PANS working criteria (National Institute of Mental Health [NIMH], 2010). Healthy age and sex-matched subjects were recruited as controls.
Methods: Thirty-four outpatients referred for PANS (mean age 9.5 years; SD 2.9, 71% male) and 25 neurotypical subjects matched for age and gender, were subjected to metabolite analysis. Serum samples were obtained from each participant and were analyzed using Nuclear Magnetic Resonance (NMR) spectroscopy. Subsequently, multivariate and univariate statistical analyses and Receiver Operator Curves (ROC) were performed.
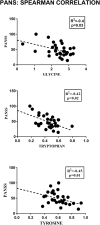
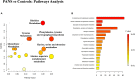
Results: Separation of the samples, in line with the presence of PANS diagnosis, was observed by applying a supervised model (R2X = 0.44, R2Y = 0.54, Q2 = 0.44, p-value < 0.0001). The significantly altered variables were 2-Hydroxybutyrate, glycine, glutamine, histidine, tryptophan. Pathway analysis indicated that phenylalanine, tyrosine, and tryptophan metabolism, as well as glutamine and glutamate metabolism, exhibited the largest deviations from neurotypical controls.
Conclusion: We found a unique plasma metabolic profile in PANS patients, significantly differing from that of healthy children, that suggests the involvement of specific patterns of neurotransmission (tryptophan, glycine, histamine/histidine) as well as a more general state of neuroinflammation and oxidative stress (glutamine, 2-Hydroxybutyrate, and tryptophan-kynurenine pathway) in the disorder. This metabolomics study offers new insights into biological mechanisms underpinning the disorder and supports research of other potential biomarkers implicated in PANS.
Keywords: biomarkers; metabolomics; neuroinflammation; oxidative stress; pediatric acute-onset neuropsychiatric syndrome.
Copyright © 2021 Murgia, Gagliano, Tanca, Or-Geva, Hendren, Carucci, Pintor, Cera, Cossu, Sotgiu, Atzori and Zuddas.
Conflict of interest statement
AG was on the advisory boards for Eli Lilly and Shire. She is/has been involved in clinical trials conducted by Eli Lilly, Shire, Lundbeck, Janssen, and Otsuka. She has been a speaker for Novartis, Eli Lilly, and Shire. SC was collaborating on projects from the European Union (7th Framework Program) and as a sub-investigator in sponsored clinical trials by Lundbeck Otsuka and Janssen Cilag. Travel support from Fidia Farmaceutici. AZ served in an advisory or consultancy role for Angelini, Lundbeck, Otsuka, and Edu-Pharma. He received conference support or speaker’s fee from Angelini, Otzuka, and Takeda. He is/has been involved in clinical trials conducted by Angelini, Roche, Lundbeck, Janssen, Servier, and Otsuka. He received royalties from Oxford University Press, and Giunti OS. SS received conference support or speaker’s fee from Bayer Pharma, Biogen Idec, Merck-Serono, Novartis, and Teva. He has been involved in clinical trials conducted by Bayer Pharma, Biogen and Teva. He received royalties from Documenta (Ed). The present work is unrelated to the above grants and relationships. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

