Role of P2X7 Receptors in Immune Responses During Neurodegeneration
- PMID: 34122013
- PMCID: PMC8187565
- DOI: 10.3389/fncel.2021.662935
Role of P2X7 Receptors in Immune Responses During Neurodegeneration
Abstract
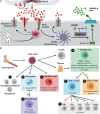
P2X7 receptors are ion-gated channels activated by ATP. Under pathological conditions, the extensive release of ATP induces sustained P2X7 receptor activation, culminating in induction of proinflammatory pathways with inflammasome assembly and cytokine release. These inflammatory conditions, whether occurring peripherally or in the central nervous system (CNS), increase blood-brain-barrier (BBB) permeability. Besides its well-known involvement in neurodegeneration and neuroinflammation, the P2X7 receptor may induce BBB disruption and chemotaxis of peripheral immune cells to the CNS, resulting in brain parenchyma infiltration. For instance, despite common effects on cytokine release, P2X7 receptor signaling is also associated with metalloproteinase secretion and activation, as well as migration and differentiation of T lymphocytes, monocytes and dendritic cells. Here we highlight that peripheral immune cells mediate the pathogenesis of Multiple Sclerosis and Parkinson's and Alzheimer's disease, mainly through T lymphocyte, neutrophil and monocyte infiltration. We propose that P2X7 receptor activation contributes to neurodegenerative disease progression beyond its known effects on the CNS. This review discusses how P2X7 receptor activation mediates responses of peripheral immune cells within the inflamed CNS, as occurring in the aforementioned diseases.
Keywords: P2X7 receptors; blood brain barrier; microglia; neurodegeneration; peripheral immune system.
Copyright © 2021 Oliveira-Giacomelli, Petiz, Andrejew, Turrini, Silva, Sack and Ulrich.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases.Int J Mol Sci. 2020 Aug 20;21(17):5996. doi: 10.3390/ijms21175996. Int J Mol Sci. 2020. PMID: 32825423 Free PMC article. Review.
-
P2X7 Receptors in Neurodegeneration: Potential Therapeutic Applications From Basic to Clinical Approaches.Front Cell Neurosci. 2021 Apr 6;15:617036. doi: 10.3389/fncel.2021.617036. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33889073 Free PMC article. Review.
-
Ion channels on microglia: therapeutic targets for neuroprotection.CNS Neurol Disord Drug Targets. 2011 Feb;10(1):44-56. doi: 10.2174/187152711794488638. CNS Neurol Disord Drug Targets. 2011. PMID: 21143139 Review.
-
The P2X7 Receptor in Inflammatory Diseases: Angel or Demon?Front Pharmacol. 2018 Feb 6;9:52. doi: 10.3389/fphar.2018.00052. eCollection 2018. Front Pharmacol. 2018. PMID: 29467654 Free PMC article. Review.
-
Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration.Front Cell Neurosci. 2017 Jul 24;11:216. doi: 10.3389/fncel.2017.00216. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28790893 Free PMC article. Review.
Cited by
-
Implication of lncRNA MSTRG.81401 in Hippocampal Pyroptosis Induced by P2X7 Receptor in Type 2 Diabetic Rats with Neuropathic Pain Combined with Depression.Int J Mol Sci. 2024 Jan 18;25(2):1186. doi: 10.3390/ijms25021186. Int J Mol Sci. 2024. PMID: 38256257 Free PMC article.
-
Calcium channel β3 subunit regulates ATP-dependent migration of dendritic cells.Sci Adv. 2023 Sep 22;9(38):eadh1653. doi: 10.1126/sciadv.adh1653. Epub 2023 Sep 20. Sci Adv. 2023. PMID: 37729408 Free PMC article.
-
VDAC1 regulates neuronal cell loss after retinal trauma injury by a mitochondria-independent pathway.Cell Death Dis. 2022 Apr 21;13(4):393. doi: 10.1038/s41419-022-04755-3. Cell Death Dis. 2022. PMID: 35449127 Free PMC article.
-
Bioinformatics insights into mitochondrial and immune gene regulation in Alzheimer's disease.Eur J Med Res. 2025 Feb 8;30(1):89. doi: 10.1186/s40001-025-02297-w. Eur J Med Res. 2025. PMID: 39920860 Free PMC article.
-
P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice.World J Gastroenterol. 2022 Aug 14;28(30):4075-4088. doi: 10.3748/wjg.v28.i30.4075. World J Gastroenterol. 2022. PMID: 36157120 Free PMC article.
References
-
- Agrawal S., Anderson P., Durbeej M., Van Rooijen N., Ivars F., Opdenakker G., et al. (2006). Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 203 1007–1016. 10.1084/jem.20051342 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

