Chemogenetic Activation of Feed-Forward Inhibitory Parvalbumin-Expressing Interneurons in the Cortico-Thalamocortical Network During Absence Seizures
- PMID: 34122016
- PMCID: PMC8193234
- DOI: 10.3389/fncel.2021.688905
Chemogenetic Activation of Feed-Forward Inhibitory Parvalbumin-Expressing Interneurons in the Cortico-Thalamocortical Network During Absence Seizures
Abstract
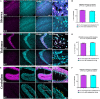
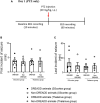
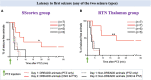
Parvalbumin-expressing (PV+) interneurons are a subset of GABAergic inhibitory interneurons that mediate feed-forward inhibition (FFI) within the cortico-thalamocortical (CTC) network of the brain. The CTC network is a reciprocal loop with connections between cortex and thalamus. FFI PV+ interneurons control the firing of principal excitatory neurons within the CTC network and prevent runaway excitation. Studies have shown that generalized spike-wave discharges (SWDs), the hallmark of absence seizures on electroencephalogram (EEG), originate within the CTC network. In the stargazer mouse model of absence epilepsy, reduced FFI is believed to contribute to absence seizure genesis as there is a specific loss of excitatory α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) at synaptic inputs to PV+ interneurons within the CTC network. However, the degree to which this deficit is directly related to seizure generation has not yet been established. Using chemogenetics and in vivo EEG recording, we recently demonstrated that functional silencing of PV+ interneurons in either the somatosensory cortex (SScortex) or the reticular thalamic nucleus (RTN) is sufficient to generate absence-SWDs. Here, we used the same approach to assess whether activating PV+ FFI interneurons within the CTC network during absence seizures would prevent or reduce seizures. To target these interneurons, mice expressing Cre recombinase in PV+ interneurons (PV-Cre) were bred with mice expressing excitatory Gq-DREADD (hM3Dq-flox) receptors. An intraperitoneal dose of pro-epileptic chemical pentylenetetrazol (PTZ) was used to induce absence seizure. The impact of activation of FFI PV+ interneurons during seizures was tested by focal injection of the "designer drug" clozapine N-oxide (CNO) into either the SScortex or the RTN thalamus. Seizures were assessed in PVCre/Gq-DREADD animals using EEG/video recordings. Overall, DREADD-mediated activation of PV+ interneurons provided anti-epileptic effects against PTZ-induced seizures. CNO activation of FFI either prevented PTZ-induced absence seizures or suppressed their severity. Furthermore, PTZ-induced tonic-clonic seizures were also reduced in severity by activation of FFI PV+ interneurons. In contrast, administration of CNO to non-DREADD wild-type control animals did not afford any protection against PTZ-induced seizures. These data demonstrate that FFI PV+ interneurons within CTC microcircuits could be a potential therapeutic target for anti-absence seizure treatment in some patients.
Keywords: DREADDs; GABAergic interneurons; absence seizures; cortico-thalamocortical; feed-forward inhibition; parvalbumin; pentylenetetrazol.
Copyright © 2021 Panthi and Leitch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

