Maturation-Dependent Differences in the Re-innervation of the Denervated Dentate Gyrus by Sprouting Associational and Commissural Mossy Cell Axons in Organotypic Tissue Cultures of Entorhinal Cortex and Hippocampus
- PMID: 34122019
- PMCID: PMC8194403
- DOI: 10.3389/fnana.2021.682383
Maturation-Dependent Differences in the Re-innervation of the Denervated Dentate Gyrus by Sprouting Associational and Commissural Mossy Cell Axons in Organotypic Tissue Cultures of Entorhinal Cortex and Hippocampus
Abstract
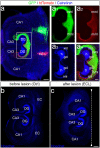
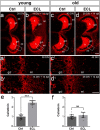
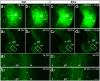
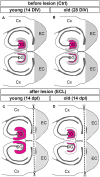
Sprouting of surviving axons is one of the major reorganization mechanisms of the injured brain contributing to a partial restoration of function. Of note, sprouting is maturation as well as age-dependent and strong in juvenile brains, moderate in adult and weak in aged brains. We have established a model system of complex organotypic tissue cultures to study sprouting in the dentate gyrus following entorhinal denervation. Entorhinal denervation performed after 2 weeks postnatally resulted in a robust, rapid, and very extensive sprouting response of commissural/associational fibers, which could be visualized using calretinin as an axonal marker. In the present study, we analyzed the effect of maturation on this form of sprouting and compared cultures denervated at 2 weeks postnatally with cultures denervated at 4 weeks postnatally. Calretinin immunofluorescence labeling as well as time-lapse imaging of virally-labeled (AAV2-hSyn1-GFP) commissural axons was employed to study the sprouting response in aged cultures. Compared to the young cultures commissural/associational sprouting was attenuated and showed a pattern similar to the one following entorhinal denervation in adult animals in vivo. We conclude that a maturation-dependent attenuation of sprouting occurs also in vitro, which now offers the chance to study, understand and influence maturation-dependent differences in brain repair in these culture preparations.
Keywords: calretinin; dentate gyrus; layer-specificity; organotypic culture; perforant path transection.
Copyright © 2021 Paul, Hildebrandt-Einfeldt, Beeg Moreno, Del Turco and Deller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Re-innervation of the Denervated Dentate Gyrus by Sprouting Associational and Commissural Mossy Cell Axons in Organotypic Tissue Cultures of Entorhinal Cortex and Hippocampus.Front Mol Neurosci. 2019 Nov 12;12:270. doi: 10.3389/fnmol.2019.00270. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31798410 Free PMC article.
-
Enhanced but delayed axonal sprouting of the commissural/associational pathway following a combined entorhinal cortex/fimbria fornix lesion.J Comp Neurol. 1995 Jan 16;351(3):453-64. doi: 10.1002/cne.903510311. J Comp Neurol. 1995. PMID: 7535807
-
Comparison of commissural sprouting in the mouse and rat fascia dentata after entorhinal cortex lesion.Hippocampus. 2003;13(6):685-99. doi: 10.1002/hipo.10118. Hippocampus. 2003. PMID: 12962314
-
Structural reorganization of the dentate gyrus following entorhinal denervation: species differences between rat and mouse.Prog Brain Res. 2007;163:501-28. doi: 10.1016/S0079-6123(07)63027-1. Prog Brain Res. 2007. PMID: 17765735 Review.
-
Cellular and molecular correlates to plasticity during recovery from injury in the developing mammalian brain.Prog Brain Res. 1996;108:365-77. doi: 10.1016/s0079-6123(08)62552-2. Prog Brain Res. 1996. PMID: 8979814 Review.
Cited by
-
Time-lapse imaging of identified granule cells in the mouse dentate gyrus after entorhinal lesion in vitro reveals heterogeneous cellular responses to denervation.Front Neuroanat. 2025 Jan 21;18:1513511. doi: 10.3389/fnana.2024.1513511. eCollection 2024. Front Neuroanat. 2025. PMID: 39906761 Free PMC article.
-
Electrophysiologically calibrated optogenetic stimulation of dentate granule cells mitigates dendritic spine loss in denervated organotypic entorhino-hippocampal slice cultures.Sci Rep. 2025 Feb 7;15(1):4563. doi: 10.1038/s41598-025-88536-w. Sci Rep. 2025. PMID: 39915664 Free PMC article.
References
-
- Askvig J. M., Watt J. A. (2019). Absence of axonal sprouting following unilateral lesion in 125-day-old rat supraoptic nucleus may be due to age-dependent decrease in protein levels of ciliary neurotrophic factor receptor alpha. J. Comp. Neurol. 527, 2291–2301. 10.1002/cne.24675 - DOI - PMC - PubMed
-
- Cotman C. W., Nadler J. V. (1978). “Reactive synaptogenesis in the hippocampus,” in Neuronal Plasticity, ed Cotman C. W. (New York, NY: Raven Press; ), 227–271.
LinkOut - more resources
Full Text Sources

