Stochasticity Versus Determinacy in Neurobiology: From Ion Channels to the Question of the "Free Will"
- PMID: 34122020
- PMCID: PMC8190656
- DOI: 10.3389/fnsys.2021.629436
Stochasticity Versus Determinacy in Neurobiology: From Ion Channels to the Question of the "Free Will"
Abstract
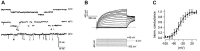
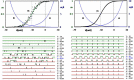
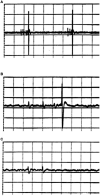
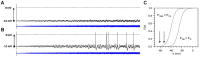
If one accepts that decisions are made by the brain and that neuronal mechanisms obey deterministic physical laws, it is hard to deny what some brain researchers postulate, such as "We do not do what we want, but we want what we do" and "We should stop talking about freedom. Our actions are determined by physical laws." This point of view has been substantially supported by spectacular neurophysiological experiments demonstrating action-related brain activity (readiness potentials, blood oxygen level-dependent signals) occurring up to several seconds before an individual becomes aware of his/her decision to perform the action. This report aims to counter the deterministic argument for the absence of free will by using experimental data, supplemented by computer simulations, to demonstrate that biological systems, specifically brain functions, are built on principle randomness, which is introduced already at the lowest level of neuronal information processing, the opening and closing of ion channels. Switching between open and closed states follows physiological laws but also makes use of randomness, which is apparently introduced by Brownian motion - principally unavoidable under all life-compatible conditions. Ion-channel stochasticity, manifested as noise, function is not smoothed out toward higher functional levels but can even be amplified by appropriate adjustment of the system's non-linearities. Examples shall be given to illustrate how stochasticity can propagate from ion channels to single neuron action potentials to neuronal network dynamics to the interactions between different brain nuclei up to the control of autonomic functions. It is proposed that this intrinsic stochasticity helps to keep the brain in a flexible state to explore diverse alternatives as a prerequisite of free decision-making.
Keywords: consciousness; noise; non-linear feedback; randomness; readiness potentials; synchronization; volitional decisions.
Copyright © 2021 Braun.
Conflict of interest statement
The author is co-owner of BM&T and main developer of the Virtual Physiology labs with benefits from the author’s experiences in education and research.
Figures

Similar articles
-
Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping.J Vis Exp. 2012 Jun 26;(64):3993. doi: 10.3791/3993. J Vis Exp. 2012. PMID: 22782131 Free PMC article.
-
Stochastic differential equation model for cerebellar granule cell excitability.PLoS Comput Biol. 2008 Feb 29;4(2):e1000004. doi: 10.1371/journal.pcbi.1000004. PLoS Comput Biol. 2008. PMID: 18463700 Free PMC article.
-
Noise from voltage-gated ion channels may influence neuronal dynamics in the entorhinal cortex.J Neurophysiol. 1998 Jul;80(1):262-9. doi: 10.1152/jn.1998.80.1.262. J Neurophysiol. 1998. PMID: 9658048
-
[The normative concept of guilt in criminal law between freedom of will and neurobiological determinism].Arch Kriminol. 2006 Nov-Dec;218(5-6):129-57. Arch Kriminol. 2006. PMID: 17217181 Review. German.
-
Consciousness, biology and quantum hypotheses.Phys Life Rev. 2012 Sep;9(3):285-94. doi: 10.1016/j.plrev.2012.07.001. Epub 2012 Jul 10. Phys Life Rev. 2012. PMID: 22925839 Review.
Cited by
-
The Constrained Disorder Principle May Account for Consciousness.Brain Sci. 2024 Feb 23;14(3):209. doi: 10.3390/brainsci14030209. Brain Sci. 2024. PMID: 38539598 Free PMC article. Review.
-
Deanthropomorphising NLP: Can a language model be conscious?PLoS One. 2024 Dec 4;19(12):e0307521. doi: 10.1371/journal.pone.0307521. eCollection 2024. PLoS One. 2024. PMID: 39631034 Free PMC article.
References
-
- Arhem P., Braun H. A., Huber M. T., Liljensrtröm H. (2005). “Dynamic state transitions in the nervous system: from ion channels to neurons to networks,” in Micro-Meso-Macro: Addressing Complex Systems Couplings, eds Liljenstrom H., Svedin U. (London: World Scientific Publication; ), 37–72. 10.1142/9789812701404_0004 - DOI
-
- Blackmore S. (2005). Conversations on Consciousness: Interviews with Twenty Minds. New York, NY: Oxford University Press.
LinkOut - more resources
Full Text Sources
Other Literature Sources

