Selective Wnt/β-Catenin Pathway Activation Concomitant With Sustained Overexpression of miR-21 is Responsible for Aristolochic Acid-Induced AKI-to-CKD Transition
- PMID: 34122087
- PMCID: PMC8193720
- DOI: 10.3389/fphar.2021.667282
Selective Wnt/β-Catenin Pathway Activation Concomitant With Sustained Overexpression of miR-21 is Responsible for Aristolochic Acid-Induced AKI-to-CKD Transition
Abstract
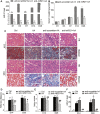
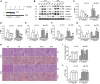
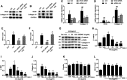
Acute kidney injury (AKI) is increasingly recognized as a cumulative risk factor for chronic kidney disease (CKD) progression. However, the underlying mechanisms remain unclear. Using an aristolochic acid (AA)-induced mouse model of AKI-to-CKD transition, we found that the development of tubulointerstitial fibrosis following AKI was accompanied with a strong activation of miR-21 and canonical Wnt signaling, whereas inhibition of miR-21 or selective silencing of Wnt ligands partially attenuated AKI-to-CKD transition. To explore the interaction between miR-21 and Wnt/β-catenin signaling, we examined the effects of genetic absence or pharmacologic inhibition of miR-21 on Wnt/β-catenin pathway expression. In miR-21-/- mice and in wild-type mice treated with anti-miR21 oligos, Wnt1 and Wnt4 canonical signaling in the renal tissue was significantly reduced, with partial reversal of renal interstitial fibrosis. Although the renal abundance of miR-21 remained unchanged after inhibition or activation of Wnt/β-catenin signaling, early intervention with ICG-001, a β-catenin inhibitor, significantly attenuated renal interstitial fibrosis. Moreover, early (within 24 h), but not late β-catenin inhibition after AA administration attenuated AA-induced apoptosis and inflammation. In conclusion, inhibition of miR-21 or β-catenin signaling may be an effective approach to prevent AKI-to-CKD progression.
Keywords: AKI-CKD transition; Wnt; aristolochic acid; miR-21; β-catenin.
Copyright © 2021 Kuang, Wu, Xue, Wang, Ding and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

