Animal and Organoid Models of Liver Fibrosis
- PMID: 34122138
- PMCID: PMC8187919
- DOI: 10.3389/fphys.2021.666138
Animal and Organoid Models of Liver Fibrosis
Abstract
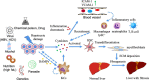
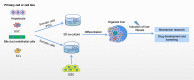
Liver fibrosis refers to the process underlying the development of chronic liver diseases, wherein liver cells are repeatedly destroyed and regenerated, which leads to an excessive deposition and abnormal distribution of the extracellular matrix such as collagen, glycoprotein and proteoglycan in the liver. Liver fibrosis thus constitutes the pathological repair response of the liver to chronic injury. Hepatic fibrosis is a key step in the progression of chronic liver disease to cirrhosis and an important factor affecting the prognosis of chronic liver disease. Further development of liver fibrosis may lead to structural disorders of the liver, nodular regeneration of hepatocytes and the formation of cirrhosis. Hepatic fibrosis is histologically reversible if treated aggressively during this period, but when fibrosis progresses to the stage of cirrhosis, reversal is very difficult, resulting in a poor prognosis. There are many causes of liver fibrosis, including liver injury caused by drugs, viral hepatitis, alcoholic liver, fatty liver and autoimmune disease. The mechanism underlying hepatic fibrosis differs among etiologies. The establishment of an appropriate animal model of liver fibrosis is not only an important basis for the in-depth study of the pathogenesis of liver fibrosis but also an important means for clinical experts to select drugs for the prevention and treatment of liver fibrosis. The present study focused on the modeling methods and fibrosis characteristics of different animal models of liver fibrosis, such as a chemical-induced liver fibrosis model, autoimmune liver fibrosis model, cholestatic liver fibrosis model, alcoholic liver fibrosis model and non-alcoholic liver fibrosis model. In addition, we also summarize the research and application prospects concerning new organoids in liver fibrosis models proposed in recent years. A suitable animal model of liver fibrosis and organoid fibrosis model that closely resemble the physiological state of the human body will provide bases for the in-depth study of the pathogenesis of liver fibrosis and the development of therapeutic drugs.
Keywords: animal; fibrosis; liver; model; organoid.
Copyright © 2021 Bao, Wang, Pan, Zhang, Chen, Xu, Mao and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

