De novo Explorations of Sarcopenia via a Dynamic Model
- PMID: 34122142
- PMCID: PMC8194405
- DOI: 10.3389/fphys.2021.670381
De novo Explorations of Sarcopenia via a Dynamic Model
Abstract
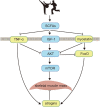
Background: The cause of sarcopenia has been observed over decades by clinical trials, which, however, are still insufficient to systematically unravel the enigma of how resistance exercise mediates skeletal muscle mass. Materials and Methods: Here, we proposed a minimal regulatory network and developed a dynamic model to rigorously investigate the mechanism of sarcopenia. Our model is consisted of eight ordinary differential equations and incorporates linear and Hill-function terms to describe positive and negative feedbacks between protein species, respectively. Results: A total of 720 samples with 10 scaled intensities were included in simulations, which revealed the expression level of AKT (maximum around 3.9-fold) and mTOR (maximum around 5.5-fold) at 3, 6, and 24 h at high intensity, and non-monotonic relation (ranging from 1.2-fold to 1.7-fold) between the graded intensities and skeletal muscle mass. Furthermore, continuous dynamics (within 24 h) of AKT, mTOR, and other proteins were obtained accordingly, and we also predicted the delaying effect with the median of maximized muscle mass shifting from 1.8-fold to 4.6-fold during a 4-fold increase of delay coefficient. Conclusion: The de novo modeling framework sheds light on the interdisciplinary methodology integrating computational approaches with experimental results, which facilitates the deeper understandings of exercise training and sarcopenia.
Keywords: gut microbiota; mathematical model; protein synthesis; resistance training; sarcopenia.
Copyright © 2021 Tao, Duan, Wang, Zeng, Fang, Yan and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Kakigi R., Yoshihara T., Ozaki H., Ogura Y., Ichinoseki-Sekine N., Kobayashi H., et al. (2014). Whey protein intake after resistance exercise activates mTOR signaling in a dose-dependent manner in human skeletal muscle. European Journal of Applied Physiology. 114 735–742. 10.1007/s00421-013-2812-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

