Zoantharian Endosymbiont Community Dynamics During a Stress Event
- PMID: 34122387
- PMCID: PMC8193574
- DOI: 10.3389/fmicb.2021.674026
Zoantharian Endosymbiont Community Dynamics During a Stress Event
Abstract
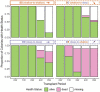
Coral reefs are complex ecosystems composed of many interacting species. One ecologically important group consists of zoantharians, which are closely related to reef-building corals. Like corals, zoantharians form mutualistic symbioses with dinoflagellate micro-algae (family Symbiodiniaceae), but their associations remain underexplored. To examine the degree to which zoantharians exhibit altered symbiont dynamics under changing environmental conditions, we reciprocally transplanted colonies of Zoanthus sansibaricus between intertidal (2 m) and subtidal (26 m) depths within a reef in Okinawa, Japan. At this location, Z. sansibaricus can associate with three Symbiodiniaceae species from two genera distributed along a light and depth gradient. We developed species-specific molecular assays and sampled colonies pre- and post-transplantation to analyze symbiont community diversity. Despite large environmental differences across depths, we detected few symbiont compositional changes resulting from transplantation stress. Colonies sourced from the intertidal zone associated with mixtures of a "shallow" Symbiodinium sp. and a "shallow" Cladocopium sp. independent of whether they were transplanted to shallow or deep waters. Colonies sourced from the subtidal zone were dominated by a "deep" Cladocopium sp. regardless of transplant depth. Subtidal colonies brought to shallow depths did not transition to the presumably high-light adapted shallow symbionts present in the new environment, but rather bleached and died. These patterns mirror observations of highly stable coral-algal associations subjected to depth transplantation. Our results indicate that Zoanthus-Symbiodiniaceae symbioses remain stable despite stress, suggesting these important reef community members have relatively low capacity to shuffle to more stress-tolerant micro-algae in response to ongoing climate change.
Keywords: Symbiodiniaceae; Zoanthus sansibaricus; light intensity; quantitative PCR; reciprocal transplant.
Copyright © 2021 Fujiwara, Kawamura, Reimer and Parkinson.
Conflict of interest statement
YF was employed by the company Nakajima Suisan Co. Ltd., while the manuscript was being written, but after field experiments had been carried out. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Baird A. H., Marshall P. A. (2002). Mortality, growth and reproduction in scleractinian corals following bleaching on the great barrier reef. Mar. Ecol. Prog. Ser. 237, 133–141. 10.3354/meps237133 - DOI
-
- Brown B., Dunne R., Goodson M., Douglas A. (2002). Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 21, 119–126. 10.1007/s00338-002-0215-z - DOI
LinkOut - more resources
Full Text Sources

