The Sterol Trafficking Pathway in Arabidopsis thaliana
- PMID: 34122463
- PMCID: PMC8187924
- DOI: 10.3389/fpls.2021.616631
The Sterol Trafficking Pathway in Arabidopsis thaliana
Abstract
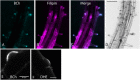
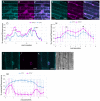
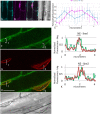
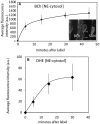
In plants, the trafficking mechanisms by which sterols move through the plant and into target cells are unknown. Earlier studies identified endosomes as primary candidates for internalization of sterols in plants, but these results have come into question. Here, we show that in elongating root cells, the internalization of sterol occurs primarily by a non-endocytic mechanism. Added fluorescent sterols [dehydroergosterol (DHE) and BODIPY-cholesterol (BCh)] do not initially label endosomes identified by fluorescent protein markers or by internalized FM4-64. Instead, the nuclear envelope, an organelle not associated with the endocytic pathway but part of the endoplasmic reticulum (ER), becomes labeled. This result is supported by experiments with the inducible overexpression of auxilin-2-like protein (AUX2 line), which blocks most endocytosis upon induction. Internalization and nuclear envelope labeling still occur in induced AUX2 cells. Longer-term incubation labels the oil body, a site involved in sterol storage. Although the first site of localization, the nuclear envelope, is part of the ER, other domains of the ER do not accumulate the label. The trafficking pathway differs from vesicular endocytosis and points toward a different pathway of sterol transport possibly involving other mechanisms, such as ER-plasma membrane contact sites and cytoplasmic transport.
Keywords: ER transport; endocytosis; nuclear envelope; oil body; sterol transport; sterol uptake.
Copyright © 2021 Kumar, Gibbs, Yeh and Griffing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Behrman E. J., Gopalan V. (2005). Cholesterol and plants. J. Chem. Educ. 82 1791–1793. 10.1021/ed082p1791 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

