Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes
- PMID: 34122886
- PMCID: PMC8152638
- DOI: 10.1039/c9sc06006e
Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes
Abstract
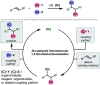
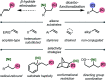
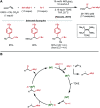
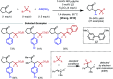
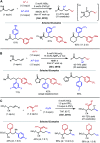
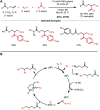
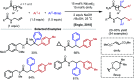
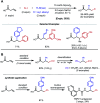
Nickel-catalyzed three-component alkene difunctionalization has rapidly emerged as a powerful tool for forging two C-C bonds in a single reaction. Building upon the powerful modes of bond construction in traditional two-component cross-coupling, various research groups have demonstrated the versatility of nickel in enabling catalytic 1,2-dicarbofunctionalization using a wide range of carbon-based electrophiles and nucleophiles and in a fully intermolecular fashion. Though this area has emerged only recently, the last few years have witnessed a proliferation of publications on this topic, underscoring the potential of this strategy to develop into a general platform that offers high regio- and stereoselectivity. This minireview highlights the recent progress in the area of intermolecular 1,2-dicarbofunctionalization of alkenes via nickel catalysis and discusses lingering challenges within this reactivity paradigm.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




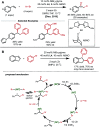
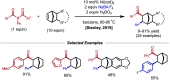
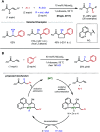
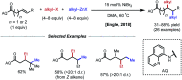


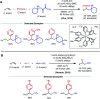
References
-
- Miyaura N. Suzuki A. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
-
-
For reviews on C(sp3)–C(sp3) cross-coupling, see:
- Netherton M. R. Fu G. C. Adv. Synth. Catal. 2004;346:1525–1532. doi: 10.1002/adsc.200404223. - DOI
- Frisch A. C. Beller M. Angew. Chem., Int. Ed. 2005;44:674–688. doi: 10.1002/anie.200461432. - DOI - PubMed
- Rudolph A. Lautens M. Angew. Chem., Int. Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611. - DOI - PubMed
- Jana R. Pathak T. P. Sigman M. S. Chem. Rev. 2011;111:1417–1492. doi: 10.1021/cr100327p. - DOI - PMC - PubMed
- Hu X. Chem. Sci. 2011;2:1867–1886. doi: 10.1039/C1SC00368B. - DOI
-
Publication types
LinkOut - more resources
Full Text Sources

