Cascade polymerizations: recent developments in the formation of polymer repeat units by cascade reactions
- PMID: 34122940
- PMCID: PMC8159232
- DOI: 10.1039/d0sc01475c
Cascade polymerizations: recent developments in the formation of polymer repeat units by cascade reactions
Abstract
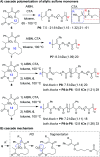
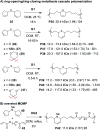
Traditionally, most polymerizations rely on simple reactions such as alkene addition, ring-opening, and condensation because they are robust, highly efficient, and selective. These reactions, however, generally only yield a single new C-C or C-O bond during each propagation step. In recent years, novel macromolecules have been prepared with propagation steps that involve cascade reactions, enabling various combinations of bond making and breaking steps to form more complex repeat units. These polymerizations are often challenging, given the requirements for high conversion and selectivity in controlled polymerizations, yet they provide polymers with unique chemical structures and significantly broaden the scope of how polymers can be made. In this perspective, we summarize the recent developments in cascade polymerizations, primarily focusing on single-component cascades (rather than multi-component polymerizations). Polymerization performance, monomer scope, and mechanisms are discussed for polymerizations utilizing radical, ionic, and metathesis-based mechanisms.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

