Ti-catalyzed ring-opening oxidative amination of methylenecyclopropanes with diazenes
- PMID: 34123005
- PMCID: PMC8159277
- DOI: 10.1039/d0sc01998d
Ti-catalyzed ring-opening oxidative amination of methylenecyclopropanes with diazenes
Abstract
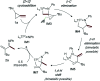
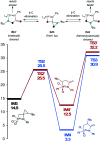
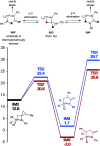
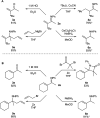
The ring-opening oxidative amination of methylenecyclopropanes (MCPs) with diazenes catalyzed by py3TiCl2(NR) complexes is reported. This reaction selectively generates branched α-methylene imines as opposed to linear α,β-unsaturated imines, which are difficult to access via other methods. Products can be isolated as the imine or hydrolyzed to the corresponding ketone in good yields. Mechanistic investigation via density functional theory suggests that the regioselectivity of these products results from a Curtin-Hammett kinetic scenario, where reversible β-carbon elimination of a spirocyclic [2 + 2] azatitanacyclobutene intermediate is followed by selectivity-determining β-hydrogen elimination of the resulting metallacycle. Further functionalizations of these branched α-methylene imine products are explored, demonstrating their utility as building blocks.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Ti-Catalyzed and -Mediated Oxidative Amination Reactions.Acc Chem Res. 2021 Sep 7;54(17):3476-3490. doi: 10.1021/acs.accounts.1c00368. Epub 2021 Aug 22. Acc Chem Res. 2021. PMID: 34420307 Free PMC article.
-
Nickel-Catalyzed Regioselective Ring-Opening Hydroarylation of Methylenecyclopropanes.Org Lett. 2025 May 23;27(20):5123-5127. doi: 10.1021/acs.orglett.5c01238. Epub 2025 May 13. Org Lett. 2025. PMID: 40357576
-
Palladium-Catalyzed Enantio- and Regioselective Ring-Opening Hydrophosphinylation of Methylenecyclopropanes.Angew Chem Int Ed Engl. 2023 Jun 26;62(26):e202303727. doi: 10.1002/anie.202303727. Epub 2023 May 16. Angew Chem Int Ed Engl. 2023. PMID: 37186017
-
Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles.Acc Chem Res. 2021 Apr 6;54(7):1766-1778. doi: 10.1021/acs.accounts.1c00027. Epub 2021 Mar 19. Acc Chem Res. 2021. PMID: 33740369 Free PMC article. Review.
-
[Novel access to indazoles based on palladium-catalyzed amination chemistry].Yakugaku Zasshi. 2008 Jul;128(7):997-1005. doi: 10.1248/yakushi.128.997. Yakugaku Zasshi. 2008. PMID: 18591867 Review. Japanese.
Cited by
-
A Mononuclear and High-Spin Tetrahedral TiII Complex.Inorg Chem. 2020 Dec 21;59(24):17834-17850. doi: 10.1021/acs.inorgchem.0c02586. Epub 2020 Dec 1. Inorg Chem. 2020. PMID: 33258366 Free PMC article.
-
Selective propargylic C(sp3)-H activation of methyl-substituted alkynes versus [2 + 2] cycloaddition at a titanium imido template.Chem Sci. 2021 Sep 28;12(41):13711-13718. doi: 10.1039/d1sc04334j. eCollection 2021 Oct 27. Chem Sci. 2021. PMID: 34760155 Free PMC article.
-
Generation of Masked TiII Intermediates from TiIV Amides via β-H Abstraction or Alkyne Deprotonation: An Example of Ti-Catalyzed Nitrene-Coupled Transfer Hydrogenation.Organometallics. 2020 Nov 9;39(21):3771-3774. doi: 10.1021/acs.organomet.0c00577. Epub 2020 Oct 20. Organometallics. 2020. PMID: 34321708 Free PMC article.
-
In Situ Catalyst Generation and Benchtop-Compatible Entry Points for TiII/TiIV Redox Catalytic Reactions.Organometallics. 2018;37(23):4439-4445. doi: 10.1021/acs.organomet.8b00474. Epub 2018 Sep 6. Organometallics. 2018. PMID: 31802785 Free PMC article.
-
Ti-Catalyzed and -Mediated Oxidative Amination Reactions.Acc Chem Res. 2021 Sep 7;54(17):3476-3490. doi: 10.1021/acs.accounts.1c00368. Epub 2021 Aug 22. Acc Chem Res. 2021. PMID: 34420307 Free PMC article.
References
-
- Park Y. Kim Y. Chang S. Chem. Rev. 2017;117:9247–9301. doi: 10.1021/acs.chemrev.6b00644. - DOI - PubMed
- Louillat M.-L. Patureau F. W. Chem. Soc. Rev. 2014;43:901–910. doi: 10.1039/C3CS60318K. - DOI - PubMed
- Zhou Y. Yuan J. Yang Q. Xiao Q. Peng Y. ChemCatChem. 2016;8:2178–2192. doi: 10.1002/cctc.201600079. - DOI
- Stokes B. J. Driver T. G. Eur. J. Org. Chem. 2011:4071–4088. doi: 10.1002/ejoc.201100150. - DOI
- Shin K. Kim H. Chang S. Acc. Chem. Res. 2015;48:1040–1052. doi: 10.1021/acs.accounts.5b00020. - DOI - PubMed
- Díaz-Requejo M. M. Pérez P. J. Chem. Rev. 2008;108:3379–3394. doi: 10.1021/cr078364y. - DOI - PubMed
- Davies H. M. L. Manning J. R. Nature. 2008;451:417–424. doi: 10.1038/nature06485. - DOI - PMC - PubMed
- Müller P. Fruit C. Chem. Rev. 2003;103:2905–2919. doi: 10.1021/cr020043t. - DOI - PubMed
- Lu H. Zhang X. P. Chem. Soc. Rev. 2011;40:1899–1909. doi: 10.1039/C0CS00070A. - DOI - PubMed
- Collet F. Lescot C. Dauban P. Chem. Soc. Rev. 2011;40:1926–1936. doi: 10.1039/C0CS00095G. - DOI - PubMed
- Halfen J. A. Curr. Org. Chem. 2005;9:657–669. doi: 10.2174/1385272053765024. - DOI
- Alderson J. M. Corbin J. R. Schomaker J. M. Acc. Chem. Res. 2017;50:2147–2158. doi: 10.1021/acs.accounts.7b00178. - DOI - PubMed
-
- Gilbert Z. W. Hue R. J. Tonks I. A. Nat. Chem. 2016;8:63–68. doi: 10.1038/nchem.2386. - DOI - PubMed
- Davis-Gilbert Z. W. Yao L. J. Tonks I. A. J. Am. Chem. Soc. 2016;138:14570–14573. doi: 10.1021/jacs.6b09939. - DOI - PMC - PubMed
- Chiu H.-C. Tonks I. A. Angew. Chem., Int. Ed. 2018;57:6090–6094. doi: 10.1002/anie.201800595. - DOI - PMC - PubMed
- Davis-Gilbert Z. W. Wen X. Goodpaster J. D. Tonks I. A. J. Am. Chem. Soc. 2018;140:7267–7281. doi: 10.1021/jacs.8b03546. - DOI - PMC - PubMed
- Davis-Gilbert Z. W. Kawakita K. Blechschmidt D. R. Tsurugi H. Mashima K. Tonks I. A. Organometallics. 2018;37:4439–4445. doi: 10.1021/acs.organomet.8b00474. - DOI - PMC - PubMed
- Chiu H.-C. See X. Y. Tonks I. A. ACS Catal. 2019;9:216–223. doi: 10.1021/acscatal.8b04669. - DOI - PMC - PubMed
- Beaumier E. P. McGreal M. E. Pancoast A. R. Wilson R. H. Moore J. T. Graziano B. J. Goodpaster J. D. Tonks I. A. ACS Catal. 2019;9:11753–11762. doi: 10.1021/acscatal.9b04107. - DOI - PMC - PubMed
- Pearce A. J. Harkins R. P. Reiner B. R. Wotal A. C. Dunscomb R. J. Tonks I. A. J. Am. Chem. Soc. 2020;142:4390–4399. doi: 10.1021/jacs.9b13173. - DOI - PMC - PubMed
- Beaumier E. P. Pearce A. J. See X. Y. Tonks I. A. Nat. Rev. Chem. 2019;3:15–34. doi: 10.1038/s41570-018-0059-x. - DOI - PMC - PubMed
- Davis-Gilbert Z. W. Tonks I. A. Dalton Trans. 2017;46:11522–11528. doi: 10.1039/C7DT02319G. - DOI - PMC - PubMed
-
- Smolensky E. Kapon M. Eisen M. S. Organometallics. 2005;24:5495–5498. doi: 10.1021/om050518h. - DOI
- Smolensky E. Kapon M. Eisen M. S. Organometallics. 2007;26:4510–4527. doi: 10.1021/om700455e. - DOI
-
; For other examples of metal-catalyzed hydroamination of MCPs see
- Nakamura I. Itagaki H. Yamamoto Y. J. Org. Chem. 1998;63:6458–6459. doi: 10.1021/jo981468b. - DOI
- Nakamura I. Itagaki H. Yamamoto Y. Chem. Heterocycl. Compd. 2001;37:1532–1540. doi: 10.1023/A:1014513411239. - DOI
- Ryu J.-S. Li G. Y. Marks T. J. J. Am. Chem. Soc. 2003;125:12584–12605. doi: 10.1021/ja035867m. - DOI - PubMed
- Shi M. Liu L.-P. Tang J. Org. Lett. 2006;8:4043–4046. doi: 10.1021/ol0614830. - DOI - PubMed
- Nishikawa D. Sakae R. Miki Y. Hirano K. Miura M. J. Org. Chem. 2016;81:12128–12134. doi: 10.1021/acs.joc.6b02483. - DOI - PubMed
- Liu Y. Ogunlana A. A. Bao X. Dalton Trans. 2018;47:5660–5669. doi: 10.1039/C8DT00131F. - DOI - PubMed
- Timmerman J. C. Robertson B. D. Widenhoefer R. A. Angew. Chem., Int. Ed. 2015;54:2251–2254. doi: 10.1002/anie.201410871. - DOI - PubMed
- Timmerman J. C. Widenhoefer R. A. Adv. Synth. Catal. 2015;357:3703–3706. doi: 10.1002/adsc.201500866. - DOI
- Shi M. Chen Y. Xu B. Org. Lett. 2003;5:1225–1228. doi: 10.1021/ol034146p. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

