A complete picture of protein unfolding and refolding in surfactants
- PMID: 34123043
- PMCID: PMC8145811
- DOI: 10.1039/c9sc04831f
A complete picture of protein unfolding and refolding in surfactants
Abstract
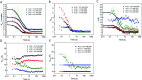
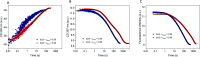
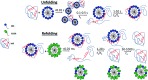
Interactions between proteins and surfactants are of relevance in many applications including food, washing powder formulations, and drug formulation. The anionic surfactant sodium dodecyl sulfate (SDS) is known to unfold globular proteins, while the non-ionic surfactant octaethyleneglycol monododecyl ether (C12E8) can be used to refold proteins from their SDS-denatured state. While unfolding have been studied in detail at the protein level, a complete picture of the interplay between protein and surfactant in these processes is lacking. This gap in our knowledge is addressed in the current work, using the β-sheet-rich globular protein β-lactoglobulin (bLG). We combined stopped-flow time-resolved SAXS, fluorescence, and circular dichroism, respectively, to provide an unprecedented in-depth picture of the different steps involved in both protein unfolding and refolding in the presence of SDS and C12E8. During unfolding, core-shell bLG-SDS complexes were formed within ∼10 ms. This involved an initial rapid process where protein and SDS formed aggregates, followed by two slower processes, where the complexes first disaggregated into single protein structures situated asymmetrically on the SDS micelles, followed by isotropic redistribution of the protein. Refolding kinetics (>100 s) were slower than unfolding (<30 s), and involved rearrangements within the mixing deadtime (∼5 ms) and transient accumulation of unfolded monomeric protein, differing in structure from the original bLG-SDS structure. Refolding of bLG involved two steps: extraction of most of the SDS from the complexes followed by protein refolding. These results reveal that surfactant-mediated unfolding and refolding of proteins are complex processes with rearrangements occurring on time scales from sub-milliseconds to minutes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
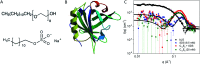




References
-
- Otzen D. E. Proteins in a brave new surfactant world. Curr. Opin. Colloid Interface Sci. 2015;20:161–169. doi: 10.1016/j.cocis.2015.07.003. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

