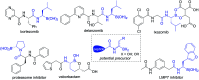
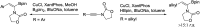Catalytic asymmetric hydrogenation of (Z)-α-dehydroamido boronate esters: direct route to alkyl-substituted α-amidoboronic esters
- PMID: 34123062
- PMCID: PMC8146211
- DOI: 10.1039/c9sc04534a
Catalytic asymmetric hydrogenation of (Z)-α-dehydroamido boronate esters: direct route to alkyl-substituted α-amidoboronic esters
Abstract
The direct catalytic asymmetric hydrogenation of (Z)-α-dehydroamino boronate esters was realized. Using this approach, a class of therapeutically relevant alkyl-substituted α-amidoboronic esters was easily synthesized in high yields with generally excellent enantioselectivities (up to 99% yield and 99% ee). The utility of the products has been demonstrated by transformation to their corresponding boronic acid derivatives by a Pd-catalyzed borylation reaction and an efficient synthesis of a potential intermediate of bortezomib. The clean, atom-economic and environment friendly nature of this catalytic asymmetric hydrogenation process would make this approach a new alternative for the production of alkyl-substituted α-amidoboronic esters of great potential in the area of organic synthesis and medicinal chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Enantioselective Rhodium-Catalyzed Hydrogenation of (Z)-N-Sulfonyl-α-dehydroamido Boronic Esters.Org Lett. 2022 Jan 21;24(2):714-719. doi: 10.1021/acs.orglett.1c04157. Epub 2022 Jan 3. Org Lett. 2022. PMID: 34978454
-
Synthesis of Chiral β-Borylated Carboxylic Esters via Nickel-Catalyzed Asymmetric Hydrogenation.Org Lett. 2019 Jun 7;21(11):3923-3926. doi: 10.1021/acs.orglett.9b00994. Epub 2019 May 22. Org Lett. 2019. PMID: 31117697
-
Rh-Catalyzed Asymmetric Hydrogenation of α,β- and β,β-Disubstituted Unsaturated Boronate Esters.Chemistry. 2020 May 12;26(27):5961-5964. doi: 10.1002/chem.202000703. Epub 2020 Apr 28. Chemistry. 2020. PMID: 32048767
-
Diastereo- and enantioselective anti-selective hydrogenation of α-amino-β-keto ester hydrochlorides and related compounds using transition-metal-chiral-bisphosphine catalysts.Chem Rec. 2014 Apr;14(2):235-50. doi: 10.1002/tcr.201300032. Epub 2014 Feb 18. Chem Rec. 2014. PMID: 24550034 Review.
-
Asymmetric hydrogenation using monodentate phosphoramidite ligands.Acc Chem Res. 2007 Dec;40(12):1267-77. doi: 10.1021/ar7001107. Epub 2007 Aug 18. Acc Chem Res. 2007. PMID: 17705446 Review.
Cited by
-
Primary trifluoroborate-iminiums enable facile access to chiral α-aminoboronic acids via Ru-catalyzed asymmetric hydrogenation and simple hydrolysis of the trifluoroborate moiety.Chem Sci. 2022 Jan 26;13(10):2946-2953. doi: 10.1039/d1sc07065g. eCollection 2022 Mar 9. Chem Sci. 2022. PMID: 35432849 Free PMC article.
-
Application of the Horner-Wadsworth-Emmons Olefination in the Construction of E-α,β-Unsaturated β-Boryl Nitriles.J Org Chem. 2025 Jun 6;90(22):7498-7506. doi: 10.1021/acs.joc.4c02915. Epub 2025 May 21. J Org Chem. 2025. PMID: 40397931 Free PMC article.
-
Enantioselective synthesis of α-aminoboronates by NiH-catalysed asymmetric hydroamidation of alkenyl boronates.Nat Commun. 2022 Sep 26;13(1):5630. doi: 10.1038/s41467-022-33411-9. Nat Commun. 2022. PMID: 36163363 Free PMC article.
-
Asymmetric hydrogenation for the synthesis of 2-substituted chiral morpholines.Chem Sci. 2021 Oct 28;12(45):15061-15066. doi: 10.1039/d1sc04288b. eCollection 2021 Nov 24. Chem Sci. 2021. PMID: 34909146 Free PMC article.
-
Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation.Chem Rev. 2022 Jan 12;122(1):269-339. doi: 10.1021/acs.chemrev.1c00496. Epub 2021 Oct 22. Chem Rev. 2022. PMID: 34677059 Free PMC article. Review.
References
-
- Kane R. C. Bross P. F. Farrell A. T. Pazdur R. Oncologist. 2003;8:508. doi: 10.1634/theoncologist.8-6-508. - DOI - PubMed
- Adams J. Kauffman M. Cancer Invest. 2004;22:304. doi: 10.1081/CNV-120030218. - DOI - PubMed
- Kane R. C. Dagher R. Farrell A. Ko C.-W. Sridhara R. Justice R. Pazdur R. Clin. Cancer Res. 2007;13:5291. doi: 10.1158/1078-0432.CCR-07-0871. - DOI - PubMed
-
-
Selected reviews, see:
- Dick L. R. Fleming P. E. Drug Discovery Today. 2010;15:243. doi: 10.1016/j.drudis.2010.01.008. - DOI - PubMed
- Baker S. J. Tomsho J. W. Benkovic S. J. Chem. Soc. Rev. 2011;40:4279. doi: 10.1039/C0CS00131G. - DOI - PubMed
- Yang W. Gao X. Wang B. Med. Res. Rev. 2003;23:346. doi: 10.1002/med.10043. - DOI - PubMed
-
-
- Valery M. D. Abed Al Aziz Q. Morris S. Mini-Rev. Med. Chem. 2004;4:1001. doi: 10.2174/1389557043403125. - DOI - PubMed
- Matteson D. S. Med. Res. Rev. 2008;28:233. doi: 10.1002/med.20105. - DOI - PubMed
- Milo L. J. Lai J. H. Wu W. Liu Y. Maw H. Li Y. Jin Z. Shu Y. Poplawski S. E. Wu Y. Sanford D. G. Sudmeier J. L. Bachovchin W. W. J. Med. Chem. 2011;54:4365. doi: 10.1021/jm200460q. - DOI - PubMed
- Micale N. Scarbaci K. Troiano V. Ettari R. Grasso S. Zappalá M. Med. Res. Rev. 2014;34:1001. doi: 10.1002/med.21312. - DOI - PubMed
-
-
Selected reviews and examples, see:
- Baker S. J. Ding C. Z. Akama T. Zhang Y.-K. Hernandez V. Xia Y. Future Med. Chem. 2009;1:1275. doi: 10.4155/fmc.09.71. - DOI - PubMed
- Han L. Wen Y. Li R. Xu B. Ge Z. Wang X. Cheng T. Cui J. Li R. Bioorg. Med. Chem. 2017;25:4031. doi: 10.1016/j.bmc.2017.05.049. - DOI - PubMed
- Markus K., Oliver H., Philipp H. and Michaul B., PCT Int. Appl., WO 2016050359 A1, 2016
- Dorsey B. D. Iqbal M. Chatterjee S. Menta E. Bernardini R. Bernareggi A. Cassarà P. G. Arasmo G. D. Ferretti E. Munari S. D. Oliva A. Pezzoni G. Allievi C. Strepponi I. Ruggeri B. Ator M. A. Williams M. Mallamo J. P. J. Med. Chem. 2008;51:1068. doi: 10.1021/jm7010589. - DOI - PubMed
- Teicher B. A. Tomaszewski J. E. Biochem. Pharmacol. 2015;96:1. doi: 10.1016/j.bcp.2015.04.008. - DOI - PubMed
- Rentsch A. Landsberg D. Brodmann T. Bülow L. Girbig A. Kalesse M. Angew. Chem., Int. Ed. 2013;52:5450. doi: 10.1002/anie.201207900. - DOI - PubMed
- Chauhan D. Tian Z. Zhou B. Kuhn D. Orlowski R. Raje N. Richardson P. Anderson K. C. Clin. Cancer Res. 2011;17:5311. doi: 10.1158/1078-0432.CCR-11-0476. - DOI - PMC - PubMed
-
-
- Awano T. Ohmura T. Suginome M. J. Am. Chem. Soc. 2011;133:20738. doi: 10.1021/ja210025q. - DOI - PubMed
- Ohmura T. Awano T. Suginome M. J. Am. Chem. Soc. 2010;132:13191. doi: 10.1021/ja106632j. - DOI - PubMed
- Ohmura T. Awano T. Suginome M. Chem. Lett. 2009;38:664. doi: 10.1246/cl.2009.664. - DOI
- Ohmura T. Miwa K. Awano T. Suginome M. Chem.—Asian J. 2018;13:2414. doi: 10.1002/asia.201800536. - DOI - PubMed
LinkOut - more resources
Full Text Sources