Reductive radical-initiated 1,2-C migration assisted by an azidyl group
- PMID: 34123076
- PMCID: PMC8163324
- DOI: 10.1039/d0sc02559c
Reductive radical-initiated 1,2-C migration assisted by an azidyl group
Abstract
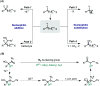
We report here a novel reductive radical-polar crossover reaction that is a reductive radical-initiated 1,2-C migration of 2-azido allyl alcohols enabled by an azidyl group. The reaction tolerates diverse migrating groups, such as alkyl, alkenyl, and aryl groups, allowing access to n+1 ring expansion of small to large rings. The possibility of directly using propargyl alcohols in one-pot is also described. Mechanistic studies indicated that an azidyl group is a good leaving group and provides a driving force for the 1,2-C migration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Cu0-Promoted Truce-Smiles Rearrangement for Aryl-Difluoromethylenation of C═C Bonds via a Reductive Radical-Polar Crossover Process.J Org Chem. 2024 Oct 4;89(19):13947-13952. doi: 10.1021/acs.joc.4c01074. Epub 2024 Sep 16. J Org Chem. 2024. PMID: 39279455
-
An Olefinic 1,2-Boryl-Migration Enabled by Radical Addition: Construction of gem-Bis(boryl)alkanes.Angew Chem Int Ed Engl. 2019 Jul 8;58(28):9448-9452. doi: 10.1002/anie.201903721. Epub 2019 May 27. Angew Chem Int Ed Engl. 2019. PMID: 31058401
-
Visible-Light Induced C(sp2 )-H Amidation with an Aryl-Alkyl σ-Bond Relocation via Redox-Neutral Radical-Polar Crossover.Angew Chem Int Ed Engl. 2021 Nov 22;60(48):25235-25240. doi: 10.1002/anie.202108775. Epub 2021 Oct 21. Angew Chem Int Ed Engl. 2021. PMID: 34558167
-
Pd-Catalyzed Isocyanide Assisted Reductive Cyclization of 1-(2-Hydroxyphenyl)-propargyl Alcohols for 2-Alkyl/Benzyl Benzofurans and Their Useful Oxidative Derivatization.J Org Chem. 2015 Dec 18;80(24):12311-20. doi: 10.1021/acs.joc.5b02204. Epub 2015 Dec 7. J Org Chem. 2015. PMID: 26599200
-
Reductive radical-polar crossover: traditional electrophiles in modern radical reactions.Chem Sci. 2019 Aug 16;10(36):8285-8291. doi: 10.1039/c9sc03359a. eCollection 2019 Sep 28. Chem Sci. 2019. PMID: 32055300 Free PMC article. Review.
References
-
- Tsunoi S. Ryu I. Yamasaki S. Tanaka M. Sonoda N. Komatsu M. Chem. Commun. 1997:1889–1890. doi: 10.1039/A704934J. - DOI
-
- Denes F. Chemla F. Normant J. F. Angew. Chem., Int. Ed. 2003;42:4043–4046. doi: 10.1002/anie.200250474. - DOI - PubMed
- Denes F. Cutri S. Perez-Luna A. Chemla F. Chem.–Eur. J. 2006;12:6506–6513. doi: 10.1002/chem.200600334. - DOI - PubMed
- Perez-Luna A. Botuha C. Ferreira F. Chemla F. Chem.–Eur. J. 2008;14:8784–8788. doi: 10.1002/chem.200801451. - DOI - PubMed
-
- Miyake Y. Nakajima K. Nishibayashi Y. J. Am. Chem. Soc. 2012;134:3338–3341. doi: 10.1021/ja211770y. - DOI - PubMed
- Kohls P. Jadhav D. Pandey G. Reiser O. Org. Lett. 2012;14:672–675. doi: 10.1021/ol202857t. - DOI - PubMed
- Yasu Y. Koike T. Akita M. Adv. Synth. Catal. 2012;354:3414–3420. doi: 10.1002/adsc.201200588. - DOI
- Chu L. Ohta C. Zuo Z. MacMillan D. W. C. J. Am. Chem. Soc. 2014;136:10886–10889. doi: 10.1021/ja505964r. - DOI - PMC - PubMed
- El Marrouni A. Ritts C. B. Balsells J. Chem. Sci. 2018;9:6639–6646. doi: 10.1039/C8SC02253D. - DOI - PMC - PubMed
-
- Yatham V. R. Shen Y. Martin R. Angew. Chem., Int. Ed. 2017;56:10915–10919. doi: 10.1002/anie.201706263. - DOI - PubMed
- Schwarz J. L. Schäfers F. Tlahuext-Aca A. Lückemeier L. Glorius F. J. Am. Chem. Soc. 2018;140:12705–12709. doi: 10.1021/jacs.8b08052. - DOI - PubMed
- Hu A. Chen Y. Guo J. Yu N. An Q. Zuo Z. J. Am. Chem. Soc. 2018;140:13580–13585. doi: 10.1021/jacs.8b08781. - DOI - PubMed
- Liao L. Cao G. Ye J. Sun G. Zhou W. Gui Y. Yan S. Shen G. Yu D. J. Am. Chem. Soc. 2018;140:17338–17342. doi: 10.1021/jacs.8b08792. - DOI - PubMed
- Ju T. Fu Q. Ye J. Zhang Z. Liao L. Yan S. Tian X. Luo S. Li J. Yu D. Angew. Chem., Int. Ed. 2018;57:13897–13901. doi: 10.1002/anie.201806874. - DOI - PubMed
- Fu Q. Bo Z. Ye J. Ju T. Huang H. Liao L. Yu D. Nat. Commun. 2019;10:3592–3600. doi: 10.1038/s41467-019-11528-8. - DOI - PMC - PubMed
- Mitsunuma H. Tanabe S. Fuse H. Ohkubo K. Kanai M. Chem. Sci. 2019;10:3459–3465. doi: 10.1039/C8SC05677C. - DOI - PMC - PubMed
- Donabauer K. Maity M. Berger A. L. Huff G. S. Crespi S. König B. Chem. Sci. 2019;10:5162–5166. doi: 10.1039/C9SC01356C. - DOI - PMC - PubMed
- Meng Q. Schirmer T. E. Berger A. L. Donabauer K. König B. J. Am. Chem. Soc. 2019;141:11393–11397. doi: 10.1021/jacs.9b05360. - DOI - PMC - PubMed
- Ni S. Padial N. M. Kingston C. Vantourout J. C. Schmitt D. C. Edwards J. T. Kruszyk M. M. Merchant R. R. Mykhailiuk P. K. Sanchez B. B. Yang S. Perry M. A. Gallego G. M. Mousseau J. J. Collins M. R. Cherney R. J. Lebed P. S. Chen J. S. Qin T. Baran P. S. J. Am. Chem. Soc. 2019;141:6726–6739. doi: 10.1021/jacs.9b02238. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources