Tubularenes
- PMID: 34123082
- PMCID: PMC8163370
- DOI: 10.1039/d0sc03384g
Tubularenes
Erratum in
- doi: 10.1039/D0SC90214D
Abstract
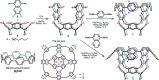
We report the synthesis and characterization of conjugated, conformationally rigid, and electroactive carbon-based nanotubes that we term tubularenes. These structures are constructed from a resorcin[n b]arene base. Cyclization of the conjugated aromatic nanotube is achieved in one-pot eight-fold C-C bond formation via Suzuki-Miyaura cross-coupling. DFT calculations indicate a buildup of strain energy in excess of 90 kcal mol-1. The resulting architectures contain large internal void spaces >260 Å3, are fluorescent, and able to accept up to 4 electrons. This represents the first scaffolding approach that provides conjugated nanotube architectures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Song N. Kakuta T. Yamagishi T.-a. Yang Y.-W. Ogoshi T. Chem. 2018;4:2029–2053.
LinkOut - more resources
Full Text Sources
Other Literature Sources

