Asymmetric synthesis of γ-chiral borylalkanes via sequential reduction/hydroboration using a single copper catalyst
- PMID: 34123150
- PMCID: PMC8163415
- DOI: 10.1039/d0sc03759a
Asymmetric synthesis of γ-chiral borylalkanes via sequential reduction/hydroboration using a single copper catalyst
Abstract
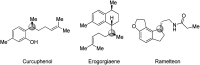
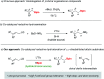
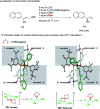
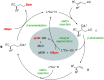
The synthesis of γ-chiral borylalkanes through copper-catalyzed enantioselective SN2'-reduction of γ,γ-disubstituted allylic substrates and subsequent hydroboration was reported. A copper-DTBM-Segphos catalyst produced a range of γ-chiral alkylboronates from easily accessible allylic acetate or benzoate with high enantioselectivities up to 99% ee. Furthermore, selective organic transformations of the resulting γ-chiral alkylboronates generated the corresponding γ-chiral alcohol, arene and amine compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Asymmetric Synthesis of Borylalkanes via Copper-Catalyzed Enantioselective Hydroallylation.J Am Chem Soc. 2016 Nov 23;138(46):15146-15149. doi: 10.1021/jacs.6b11229. Epub 2016 Nov 9. J Am Chem Soc. 2016. PMID: 27808507
-
Enantioselective Copper-Catalyzed Ring-Opening Diboration of Arylidenecyclopropanes to Access Chiral Skipped 1,4- and 1,3-Diboronates.J Am Chem Soc. 2024 Feb 28;146(8):5366-5374. doi: 10.1021/jacs.3c12675. Epub 2024 Feb 14. J Am Chem Soc. 2024. PMID: 38354313
-
Development of biisoquinoline-based chiral diaminocarbene ligands: enantioselective SN2' allylic alkylation catalyzed by copper-carbene complexes.J Org Chem. 2008 Mar 7;73(5):1983-6. doi: 10.1021/jo702512z. Epub 2008 Feb 12. J Org Chem. 2008. PMID: 18266387
-
Palladium(II)-Catalyzed Enantioselective Reactions Using COP Catalysts.Acc Chem Res. 2016 Oct 18;49(10):2220-2231. doi: 10.1021/acs.accounts.6b00398. Epub 2016 Sep 30. Acc Chem Res. 2016. PMID: 27689745
-
[Synthesis and application of novel biaryl compounds with axial chirality as catalysts in enantioselective reactions].Yakugaku Zasshi. 2000 Jan;120(1):68-75. doi: 10.1248/yakushi1947.120.1_68. Yakugaku Zasshi. 2000. PMID: 10655783 Review. Japanese.
Cited by
-
Enantioselective synthesis of γ-chiral amides via copper-catalyzed reductive relay hydroaminocarbonylation.Nat Commun. 2024 Aug 7;15(1):6705. doi: 10.1038/s41467-024-51048-8. Nat Commun. 2024. PMID: 39112513 Free PMC article.
References
-
- Werner E. W. Mei T.-S. Burckle A. J. Sigman M. S. Science. 2012;338:1455. doi: 10.1126/science.1229208. - DOI - PMC - PubMed
- Mei T.-S. Patel H. H. Sigman M. S. Nature. 2014;508:340. doi: 10.1038/nature13231. - DOI - PMC - PubMed
- Liu W.-B. Okamoto N. Alexy E. J. Hong A. Y. Tran K. Stoltz B. M. J. Am. Chem. Soc. 2016;138:5234. doi: 10.1021/jacs.6b02153. - DOI - PMC - PubMed
- Wang Z.-X. Bai X.-Y. Yao H.-C. Li B.-J. J. Am. Chem. Soc. 2016;138:14872. doi: 10.1021/jacs.6b10415. - DOI - PubMed
- Liu J. Yuan Q. Toste F. D. Sigman M. S. Nat. Chem. 2019;11:710. doi: 10.1038/s41557-019-0289-7. - DOI - PMC - PubMed
-
- Ito H. Ito S. Sasaki Y. Matsuura K. Sawamura M. J. Am. Chem. Soc. 2007;129:14856. doi: 10.1021/ja076634o. - DOI - PubMed
- Ito H. Kosaka Y. Nonoyama K. Sasaki Y. Sawamura M. Angew. Chem., Int. Ed. 2008;47:7424. doi: 10.1002/anie.200802342. - DOI - PubMed
- Lee Y. Hoveyda A. H. J. Am. Chem. Soc. 2009;131:3160. doi: 10.1021/ja809382c. - DOI - PMC - PubMed
- Matsuda N. Hirano K. Satoh T. Miura M. J. Am. Chem. Soc. 2013;135:4934. doi: 10.1021/ja4007645. - DOI - PubMed
- Meng F. McGrath K. P. Hoveyda A. H. Nature. 2014;513:367. doi: 10.1038/nature13735. - DOI - PMC - PubMed
- Kubota K. Yamamoto E. Ito H. J. Am. Chem. Soc. 2015;137:420. doi: 10.1021/ja511247z. - DOI - PubMed
- Jia T. Cao P. Wang B. Lou Y. Yin X. Wang M. Liao J. J. Am. Chem. Soc. 2015;137:13760. doi: 10.1021/jacs.5b09146. - DOI - PubMed
- Nishikawa D. Hirano K. Miura M. J. Am. Chem. Soc. 2015;137:15620. doi: 10.1021/jacs.5b09773. - DOI - PubMed
- Meng F. Li X. Torker S. Shi Y. Shen X. Hoveyda A. H. Nature. 2016;537:387. doi: 10.1038/nature19063. - DOI - PMC - PubMed
- Yeung K. Ruscoe R. E. Rae J. Pulis A. P. Procter D. J. Angew. Chem., Int. Ed. 2016;55:11912. doi: 10.1002/anie.201606710. - DOI - PMC - PubMed
- Jang H. Romiti F. Torker S. Hoveyda A. H. Nat. Chem. 2017;9:1269. doi: 10.1038/nchem.2816. - DOI - PMC - PubMed
- Logan K. M. Brown M. K. Angew. Chem., Int. Ed. 2017;56:851. doi: 10.1002/anie.201609844. - DOI - PMC - PubMed
- Huang Y. Smith K. B. Brown M. K. Angew. Chem., Int. Ed. 2017;56:13314. doi: 10.1002/anie.201707323. - DOI - PMC - PubMed
- Lee J. Radomkit S. Torker S. del Pozo J. Hoveyda A. H. Nat. Chem. 2018;10:99. doi: 10.1038/nchem.2861. - DOI - PMC - PubMed
- Itoh T. Kanzaki Y. Shimizu Y. Kanai M. Angew. Chem., Int. Ed. 2018;57:8265. doi: 10.1002/anie.201804117. - DOI - PubMed
LinkOut - more resources
Full Text Sources

