Conformation control through concurrent N-H⋯S and N-H⋯O[double bond, length as m-dash]C hydrogen bonding and hyperconjugation effects
- PMID: 34123167
- PMCID: PMC8163419
- DOI: 10.1039/d0sc03339a
Conformation control through concurrent N-H⋯S and N-H⋯O[double bond, length as m-dash]C hydrogen bonding and hyperconjugation effects
Abstract
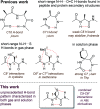
In addition to the classical N-H⋯O[double bond, length as m-dash]C non-covalent interaction, less conventional types of hydrogen bonding, such as N-H⋯S, may play a key role in determining the molecular structure. In this work, using theoretical calculations in combination with spectroscopic analysis in both gas phase and solution phase, we demonstrate that both these H-bonding modes exist simultaneously in low-energy conformers of capped derivatives of Attc, a thietane α-amino acid. 6-Membered ring inter-residue N-H⋯S interactions (C6γ), assisted by hyperconjugation between the thietane ring and the backbone, combine with 5-membered ring intra-residue backbone N-H⋯O[double bond, length as m-dash]C interactions (C5) to provide a C5-C6γ feature that stabilizes a planar geometry in the monomer unit. Two contiguous C5-C6γ features in the planar dimer implicate an unprecedented three-centre H-bond of the type C[double bond, length as m-dash]O⋯H(N)⋯SR2, while the trimer adopts two C5-C6γ features separated by a Ramachandran α-type backbone configuration. These low-energy conformers are fully characterized in the gas phase and support is presented for their existence in solution state.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Gilli G. and Gilli P., The Nature of the Hydrogen Bond. Outline of a Comprehensive Hydrogen Bond Theory, Oxford University Press, New-York, USA, 2009
-
- Pihko P. M., Hydrogen Bonding in Organic Synthesis, Wiley-VCH, Weinhein, Germany, 2009
-
- Burrows A. D., in Supramolecular Assembly Via Hydrogen Bonds I, ed. D. M. P. Mingos, 2004, vol. 108, pp. 55–95
-
- Braga D., Maini L., Polito M. and Grepioni F., in Supramolecular Assembly Via Hydrogen Bonds II, ed. D. M. P. Mingos, 2004, vol. 111, pp. 1–32
-
- Hecht S. and Huc I., Foldamers: Structure, Properties, and Applications, Wiley-VCH, Weinheim, Germany, 2007
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

