Prediction and mitigation of mutation threats to COVID-19 vaccines and antibody therapies
- PMID: 34123321
- PMCID: PMC8153213
- DOI: 10.1039/d1sc01203g
Prediction and mitigation of mutation threats to COVID-19 vaccines and antibody therapies
Abstract
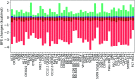
Antibody therapeutics and vaccines are among our last resort to end the raging COVID-19 pandemic. They, however, are prone to over 5000 mutations on the spike (S) protein uncovered by a Mutation Tracker based on over 200 000 genome isolates. It is imperative to understand how mutations will impact vaccines and antibodies in development. In this work, we first study the mechanism, frequency, and ratio of mutations on the S protein which is the common target of most COVID-19 vaccines and antibody therapies. Additionally, we build a library of 56 antibody structures and analyze their 2D and 3D characteristics. Moreover, we predict the mutation-induced binding free energy (BFE) changes for the complexes of S protein and antibodies or ACE2. By integrating genetics, biophysics, deep learning, and algebraic topology, we reveal that most of the 462 mutations on the receptor-binding domain (RBD) will weaken the binding of S protein and antibodies and disrupt the efficacy and reliability of antibody therapies and vaccines. A list of 31 antibody disrupting mutants is identified, while many other disruptive mutations are detailed as well. We also unveil that about 65% of the existing RBD mutations, including those variants recently found in the United Kingdom (UK) and South Africa, will strengthen the binding between the S protein and human angiotensin-converting enzyme 2 (ACE2), resulting in more infectious COVID-19 variants. We discover the disparity between the extreme values of RBD mutation-induced BFE strengthening and weakening of the bindings with antibodies and angiotensin-converting enzyme 2 (ACE2), suggesting that SARS-CoV-2 is at an advanced stage of evolution for human infection, while the human immune system is able to produce optimized antibodies. This discovery, unfortunately, implies the vulnerability of current vaccines and antibody drugs to new mutations. Our predictions were validated by comparison with more than 1400 deep mutations on the S protein RBD. Our results show the urgent need to develop new mutation-resistant vaccines and antibodies and to prepare for seasonal vaccinations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Update of
-
Prediction and mitigation of mutation threats to COVID-19 vaccines and antibody therapies.ArXiv [Preprint]. 2021 Mar 9:arXiv:2010.06357v2. ArXiv. 2021. Update in: Chem Sci. 2021 Apr 13;12(20):6929-6948. doi: 10.1039/d1sc01203g. PMID: 33083498 Free PMC article. Updated. Preprint.
References
-
- Shin M. D. Shukla S. Chung Y. H. Beiss V. Chan S. K. Ortega-Rivera O. A. Wirth D. M. Chen A. Sack M. Pokorski J. K. et al., COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020:1–10. - PubMed
-
- Day M., Covid-19: four fifths of cases are asymptomatic, China figures indicate, 2020 - PubMed
-
- Long Q.-X. Tang X.-J. Shi Q.-L. Li Q. Deng H.-J. Yuan J. Hu J.-L. Xu W. Zhang Y. Lv F.-J. et al., Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

