Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems
- PMID: 34123879
- PMCID: PMC8188986
- DOI: 10.3389/fcimb.2021.691210
Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems
Abstract
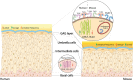
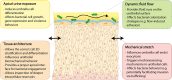
Urinary tract infections (UTIs) are among the most common infectious diseases worldwide but are significantly understudied. Uropathogenic E. coli (UPEC) accounts for a significant proportion of UTI, but a large number of other species can infect the urinary tract, each of which will have unique host-pathogen interactions with the bladder environment. Given the substantial economic burden of UTI and its increasing antibiotic resistance, there is an urgent need to better understand UTI pathophysiology - especially its tendency to relapse and recur. Most models developed to date use murine infection; few human-relevant models exist. Of these, the majority of in vitro UTI models have utilized cells in static culture, but UTI needs to be studied in the context of the unique aspects of the bladder's biophysical environment (e.g., tissue architecture, urine, fluid flow, and stretch). In this review, we summarize the complexities of recurrent UTI, critically assess current infection models and discuss potential improvements. More advanced human cell-based in vitro models have the potential to enable a better understanding of the etiology of UTI disease and to provide a complementary platform alongside animals for drug screening and the search for better treatments.
Keywords: in vitro infection model systems; microphysiological systems; mouse models; organ-on-chip; organoid; urinary tract infection (UTI); uropathogenic E. coli (UPEC); urothelium.
Copyright © 2021 Murray, Flores, Williams, Flusberg, Marr, Kwiatkowska, Charest, Isenberg and Rohn.
Conflict of interest statement
Authors CW, DF, EM, BC, and JC were or are currently employed by The Charles Stark Draper Laboratory, Inc., a not-for-profit research and development organization that develops hardware for advanced biological models. JR and BM have received research funding from AtoCap Ltd., a University College London spinoff company, to develop novel cures for urinary tract infection and bladder cancer, and JR has share options in the company. JR and CF have also received basic research funding from Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Andersen T. E., Khandige S., Madelung M., Brewer J., Kolmos H. J., Møller-Jensen J. (2012). Escherichia Coli Uropathogenesis In Vitro: Invasion, Cellular Escape, and Secondary Infection Analyzed in a Human Bladder Cell Infection Model. Infection Immun. 80, 1858–1867. 10.1128/IAI.06075-11 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

