Assessment of Volume Status and Fluid Responsiveness in Small Animals
- PMID: 34124213
- PMCID: PMC8193042
- DOI: 10.3389/fvets.2021.630643
Assessment of Volume Status and Fluid Responsiveness in Small Animals
Abstract
Intravenous fluids are an essential component of shock management in human and veterinary emergency and critical care to increase cardiac output and improve tissue perfusion. Unfortunately, there are very few evidence-based guidelines to help direct fluid therapy in the clinical setting. Giving insufficient fluids and/or administering fluids too slowly to hypotensive patients with hypovolemia can contribute to continued hypoperfusion and increased morbidity and mortality. Similarly, giving excessive fluids to a volume unresponsive patient can contribute to volume overload and can equally increase morbidity and mortality. Therefore, assessing a patient's volume status and fluid responsiveness, and monitoring patient's response to fluid administration is critical in maintaining the balance between meeting a patient's fluid needs vs. contributing to complications of volume overload. This article will focus on the physiology behind fluid responsiveness and the methodologies used to estimate volume status and fluid responsiveness in the clinical setting.
Keywords: POCUS; cats; dogs; dynamic; fluid responsiveness; static; volume status; wet lung.
Copyright © 2021 Boysen and Gommeren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
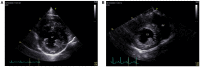
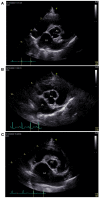
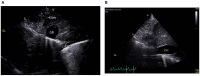
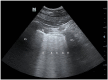

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

