Development of a Chemical Reproductive Aging Model in Female Rats
- PMID: 34124295
- PMCID: PMC8160544
- DOI: 10.21769/BioProtoc.3994
Development of a Chemical Reproductive Aging Model in Female Rats
Abstract
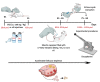
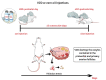
Women are born with an abundant but finite pool of ovarian follicles, which naturally and progressively decreased during their reproductive years until menstrual periods stop permanently (menopause). Perimenopause represents the transition from reproductive to non-reproductive life. It is usually characterized by neuroendocrine, metabolic and behavioral changes, which result from a follicular depletion and reduced number of ovarian follicles. During this period, around 45-50 years old, women are more likely to express mood disorders, anxiety, irritability and vasomotor symptoms. The current animal models of reproductive aging do not successfully replicate human perimenopause and the gradual changes that occur in this phase. While the traditional rat model of menopause involves ovariectomy or surgical menopause consisting of the rapid and definitive removal of the ovaries resulting in a complete loss of all ovarian hormones, natural or transitional menopause is achieved by the selective loss of ovarian follicles (perimenopause period). However, the natural aging rodent (around 18-24 months) model fails to reach very low estrogen concentrations and overlaps the processes of somatic and reproductive aging. The chronic exposure of young rodents to 4-vinylcyclohexene diepoxide (VCD) is a well-established experimental model for perimenopause and menopause studies. VCD induces loss of ovarian small follicles (primary and primordial) in mice and rats by accelerating the natural process of atresia (apoptosis). The VCD, ovary-intact or accelerated ovarian failure (AOF) model is the experimental model that most closely matches natural human progression to menopause mimicking both hormonal and behavioral changes typically manifested by women in perimenopause. Graphical abstract: The female reproductive system is regulated by a series of neuroendocrine events controlled by central and peripheral components. (A). The mechanisms involved in this control are extremely complex and have not yet been fully clarified. In female mammals whose ovulation (the most important event in a reproductive cycle) occurs spontaneously, reproductive success is achieved through the precise functional and temporal integration of the hypothalamus-pituitary-ovary (HPO) axis. (B). In women, loss of fertility appears to be primarily associated with exhaustion of ovarian follicles, and this process occurs progressively until complete follicular exhaustion marked by the final menstrual period (FMP). (C). While in female rodents, reproductive aging seems to begin as a neuroendocrine process, in which changes in hypothalamic/pituitary function appear independently of follicular atresia. The traditional rat model of menopause, ovariectomy or surgical menopause consists of the rapid and definitive removal of the ovaries resulting in a complete loss of all ovarian hormones. (D). The chronic exposure (15-30 days) to the chemical compound 4-vinylcyclehexene diepoxide (VCD) in young rodents accelerates gradual failure of ovarian function by progressive depletion of primordial and primary follicles, but retains residual ovarian tissue before brain alterations that occurs in women in perimenopause. Low doses of VCD cause the selective destruction of the small preantral follicles of the ovary without affecting other peripheral tissues.
Keywords: 4-vinylcyclohexene diepoxide (VCD); Accelerated ovarian failure (AOF); Chemical reproductive aging; Follicular depletion; Perimenopause.
Copyright © 2021 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsThe authors declare that they have no conflict of interest.
Figures


Similar articles
-
Hormonal changes and increased anxiety-like behavior in a perimenopause-animal model induced by 4-vinylcyclohexene diepoxide (VCD) in female rats.Psychoneuroendocrinology. 2014 Nov;49:130-40. doi: 10.1016/j.psyneuen.2014.06.019. Epub 2014 Jul 12. Psychoneuroendocrinology. 2014. PMID: 25080405
-
Accelerated ovarian failure: a novel, chemically induced animal model of menopause.Brain Res. 2011 Mar 16;1379:176-87. doi: 10.1016/j.brainres.2010.12.064. Epub 2011 Jan 4. Brain Res. 2011. PMID: 21211517 Free PMC article. Review.
-
Reproductive potential in the older woman.Fertil Steril. 1986 Dec;46(6):989-1001. doi: 10.1016/s0015-0282(16)49869-9. Fertil Steril. 1986. PMID: 3536609 Review.
-
Conditions and possible mechanisms of VCD-induced ovarian failure.Altern Lab Anim. 2015 Dec;43(6):385-92. doi: 10.1177/026119291504300606. Altern Lab Anim. 2015. PMID: 26753941
-
Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system.Endocrinology. 2009 Sep;150(9):4248-59. doi: 10.1210/en.2008-1802. Epub 2009 May 21. Endocrinology. 2009. PMID: 19470706 Free PMC article.
Cited by
-
Dehydroepiandrosterone modulates the PTEN/PI3K/AKT signaling pathway to alleviate 4-vinylcyclohexene diepoxide-induced premature ovarian insufficiency in rats.Exp Anim. 2024 Jul 9;73(3):319-335. doi: 10.1538/expanim.23-0179. Epub 2024 Mar 16. Exp Anim. 2024. PMID: 38494723 Free PMC article.
-
Effects of 4-vinylcyclohexene diepoxide on the cell cycle, apoptosis, and steroid hormone secretion of goat ovarian granulosa cells.In Vitro Cell Dev Biol Anim. 2022 Mar;58(3):220-231. doi: 10.1007/s11626-022-00663-0. Epub 2022 Apr 6. In Vitro Cell Dev Biol Anim. 2022. PMID: 35386089
References
-
- Bacon J. L.(2017). The Menopausal Transition. Obstet Gynecol Clin N Am 44(2): 285-296. - PubMed
-
- Brinton R. D., Gore A. C., Schmidt P. J. and Morrison J. H.(2009). Hormones, Brain and Behavior(2nd edition). In: Pfaff, D. W. et al.(Eds.). Elsevier; 2199-2222.
-
- Brinton R. D.(2010). In: Brockelhurst's Textbook of Geriatric Medicine and Gerontology. Fillit, H., Rockwood, K. and Young, J.(Eds.). Saunders 163-171.
LinkOut - more resources
Full Text Sources

