FRET-Based Genetically Encoded Sensor to Monitor Silver Ions
- PMID: 34124439
- PMCID: PMC8190795
- DOI: 10.1021/acsomega.1c00741
FRET-Based Genetically Encoded Sensor to Monitor Silver Ions
Abstract
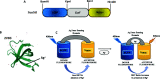
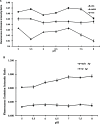
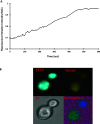
Silver is commonly used in wound dressing, photography, health care products, laboratories, pharmacy, biomedical devices, and several industrial purposes. Silver (Ag+) ions are more toxic pollutants widely scattered in the open environment by natural processes and dispersed in soil, air, and water bodies. Ag+ binds with metallothionein, macroglobulins, and albumins, which may lead to the alteration of various enzymatic metabolic pathways. To analyze the uptake and metabolism of silver ions in vitro as well as in cells, a range of high-affinity fluorescence-based nanosensors has been constructed using a periplasmic protein CusF, a part of the CusCFBA efflux complex, which is involved in providing resistance against copper and silver ions in Escherichia coli. This nanosensor was constructed by combining of two fluorescent proteins (donor and acceptor) at the N- and C-terminus of the silver-binding protein (CusF), respectively. SenSil (WT) with a binding constant (K d) of 5.171 μM was more efficient than its mutant variants (H36D and F71W). This nanosensor allows monitoring the level of silver ions in real time in prokaryotes and eukaryotes without any disruption of cells or tissues.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Bury N. R.; McGeer J. C.; Wood C. M. Effects of altering freshwater chemistry on physiological responses of rainbow trout to silver exposure. Environ. Toxicol. Chem. 1999, 18, 49–55. 10.1002/etc.5620180107. - DOI
-
- Krizkova S.; Adam V.; Kizek R. Phytotoxicity of silver ions. Chem. Listy 2009, 103, 559–568.
LinkOut - more resources
Full Text Sources

