Ion shuttling between emulsion droplets by crown ether modified gold nanoparticles
- PMID: 34124578
- PMCID: PMC8168925
- DOI: 10.1039/d1na00009h
Ion shuttling between emulsion droplets by crown ether modified gold nanoparticles
Abstract
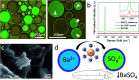
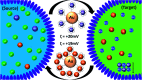
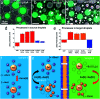
Selective unidirectional transport of barium ions between droplets in a water-in-chloroform emulsion is demonstrated. Gold nanoparticles (GNPs) modified with a thiolated crown ether act as barium ion complexing shuttles that carry the ions from one population of droplets (source) to another (target). This process is driven by a steep barium ion concentration gradient between source and target droplets. The concentration of barium ions in the target droplets is kept low at all times by the precipitation of insoluble barium sulfate. A potential role of electrostatically coupled secondary processes that maintain the electroneutrality of the emulsion droplets is discussed. Charging of the GNP metal cores by electron transfer in the presence of the Fe(ii)/Fe(iii) redox couple appears to affect the partitioning of the GNPs between the water droplets and the chloroform phase. Processes have been monitored and studied by optical microscopy, Raman spectroscopy, cryogenic scanning electron microscopy (cryo-SEM) and zeta potential. The shuttle action of the GNPs has further been demonstrated electrochemically in a model system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Design of artificial membrane transporters from gold nanoparticles with controllable hydrophobicity.Faraday Discuss. 2016 Oct 6;191:495-510. doi: 10.1039/c6fd00037a. Epub 2016 Jul 15. Faraday Discuss. 2016. PMID: 27420179
-
The fabrication of patterned gold nanoparticle arrays via selective ion irradiation and plasma treatment.J Nanosci Nanotechnol. 2014 Aug;14(8):6158-61. doi: 10.1166/jnn.2014.8778. J Nanosci Nanotechnol. 2014. PMID: 25936078
-
Metal-Crown Ether-Porphyrin Decorated Gold Nanoparticles as High Sensitive Raman Ion Probe.J Nanosci Nanotechnol. 2018 Apr 1;18(4):2562-2568. doi: 10.1166/jnn.2018.14268. J Nanosci Nanotechnol. 2018. PMID: 29442927
-
Completely dispersible PEGylated gold nanoparticles under physiological conditions: modification of gold nanoparticles with precisely controlled PEG-b-polyamine.Langmuir. 2008 May 6;24(9):5010-7. doi: 10.1021/la703813f. Epub 2008 Apr 4. Langmuir. 2008. PMID: 18386943
-
Gold Nanoparticles Conjugated with Glycopeptides for Lectin Detection and Imaging on Cell Surface.Protein Pept Lett. 2018;25(1):84-89. doi: 10.2174/0929866525666171218124434. Protein Pept Lett. 2018. PMID: 29256341
Cited by
-
Solvatochromism of Amphiphilic Au25(SR)18 Nanoclusters Based on Supramolecular Ligand-Thiolated Crown Ether.J Phys Chem Lett. 2025 Jul 24;16(29):7331-7336. doi: 10.1021/acs.jpclett.5c01543. Epub 2025 Jul 12. J Phys Chem Lett. 2025. PMID: 40650636 Free PMC article.
-
Crown Ether-Capped Gold Nanoclusters as a Multimodal Platform for Bioimaging.ACS Omega. 2023 Mar 13;8(12):11503-11511. doi: 10.1021/acsomega.3c00426. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008092 Free PMC article.
References
-
- Molecular machines and motors, ed. J. P Sauvage, Springer Science, 2001
LinkOut - more resources
Full Text Sources

