Comparison of three air samplers for the collection of four nebulized respiratory viruses - Collection of respiratory viruses from air
- PMID: 34124803
- PMCID: PMC8530848
- DOI: 10.1111/ina.12875
Comparison of three air samplers for the collection of four nebulized respiratory viruses - Collection of respiratory viruses from air
Abstract
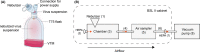
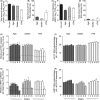
Viral respiratory tract infections are a leading cause of morbidity and mortality worldwide. Unfortunately, the transmission routes and shedding kinetics of respiratory viruses remain poorly understood. Air sampling techniques to quantify infectious viruses in the air are indispensable to improve intervention strategies to control and prevent spreading of respiratory viruses. Here, the collection of infectious virus with the six-stage Andersen cascade impactor was optimized with semi-solid gelatin as collection surface. Subsequently, the collection efficiency of the cascade impactor, the SKC BioSampler, and an in-house developed electrostatic precipitator was compared. In an in vitro set-up, influenza A virus, human metapneumovirus, parainfluenza virus type 3, and respiratory syncytial virus were nebulized and the amount of collected infectious virus and viral RNA was quantified with each air sampler. Whereas only low amounts of virus were collected using the electrostatic precipitator, high amounts were collected with the BioSampler and cascade impactor. The BioSampler allowed straight-forward sampling in liquid medium, whereas the more laborious cascade impactor allowed size fractionation of virus-containing particles. Depending on the research question, either the BioSampler or the cascade impactor can be applied in laboratory and field settings, such as hospitals to gain more insight into the transmission routes of respiratory viruses.
Keywords: air sampling; collection efficiency; electrostatic precipitator; impactor; impinger.
© 2021 The Authors. Indoor Air published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization . The top 10 causes of death. 2019. https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death. Accessed January 12, 2021.
-
- Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191‐1210. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

