Plant neighbour-modulated susceptibility to pathogens in intraspecific mixtures
- PMID: 34125197
- PMCID: PMC8483782
- DOI: 10.1093/jxb/erab277
Plant neighbour-modulated susceptibility to pathogens in intraspecific mixtures
Abstract
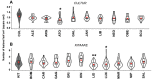
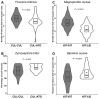
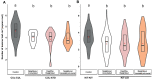
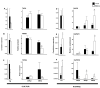
As part of a trend towards diversifying cultivated areas, varietal mixtures are subject to renewed interest as a means to manage diseases. Besides the epidemiological effects of varietal mixtures on pathogen propagation, little is known about the effect of intraspecific plant-plant interactions and their impact on responses to disease. In this study, genotypes of rice (Oryza sativa) or durum wheat (Triticum turgidum) were grown with different conspecific neighbours and manually inoculated under conditions preventing pathogen propagation. Disease susceptibility was measured together with the expression of basal immunity genes as part of the response to intra-specific neighbours. The results showed that in many cases for both rice and wheat susceptibility to pathogens and immunity was modified by the presence of intraspecific neighbours. This phenomenon, which we term 'neighbour-modulated susceptibility' (NMS), could be caused by the production of below-ground signals and does not require the neighbours to be infected. Our results suggest that the mechanisms responsible for reducing disease in varietal mixtures in the field need to be re-examined.
Keywords: Oryza sativa; Triticum turgidum; Disease; immunity; intraspecific mixture; neighbour; plant–plant interactions; rice; wheat.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures

References
-
- Ali S, Ganai BA, Kamili AN, et al. . 2018. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiological Research 212–213, 29–37. - PubMed
-
- Ballaré CL, Pierik R. 2017. The shade-avoidance syndrome: multiple signals and ecological consequences. Plant, Cell & Environment 40, 2530–2543. - PubMed
-
- Ballini E, Tavaud M, Ducasse A, et al. . 2020. Genome wide association mapping for resistance to multiple fungal pathogens in a panel issued from a broad composite cross-population of tetraploid wheat Triticum turgidum. Euphytica 216, 92.
-
- Barot S, Allard V, Cantarel A, Enjalbert J, Gauffreteau A, Goldringer I, Lata J-C, Le Roux X, Niboyet A, Porcher E. 2017. Designing mixtures of varieties for multifunctional agriculture with the help of ecology. A review. Agronomy for Sustainable Development 37, 13.
-
- Berruyer R, Adreit H, Milazzo J, Gaillard S, Berger A, Dioh W, Lebrun MH, Tharreau D. 2003. Identification and fine mapping of Pi33, the rice resistance gene corresponding to the Magnaporthe grisea avirulence gene ACE1. Theoretical and Applied Genetics 107, 1139–1147. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

