Ang2 inhibitors and Tie2 activators: potential therapeutics in perioperative treatment of early stage cancer
- PMID: 34125494
- PMCID: PMC8261516
- DOI: 10.15252/emmm.201708253
Ang2 inhibitors and Tie2 activators: potential therapeutics in perioperative treatment of early stage cancer
Abstract
Anti-angiogenic drugs targeting the VEGF pathway are most effective in advanced metastatic disease settings of certain types of cancers, whereas they have been unsuccessful as adjuvant therapies of micrometastatic disease in numerous phase III trials involving early-stage (resectable) cancers. Newer investigational anti-angiogenic drugs have been designed to inhibit the Angiopoietin (Ang)-Tie pathway. Acting through Tie2 receptors, the Ang1 ligand is a gatekeeper of endothelial quiescence. Ang2 is a dynamically expressed pro-angiogenic destabilizer of endothelium, and its upregulation is associated with poor prognosis in cancer. Besides using Ang2 blockers as inhibitors of tumor angiogenesis, little attention has been paid to their use as stabilizers of blood vessels to suppress tumor cell extravasation and metastasis. In clinical trials, Ang2 blockers have shown limited efficacy in advanced metastatic disease settings. This review summarizes preclinical evidence suggesting the potential utility of Ang2 inhibitors or Tie2 activators as neoadjuvant or adjuvant therapies in the prevention or treatment of early-stage micrometastatic disease. We further discuss the rationale and potential of combining these strategies with immunotherapy, including immune checkpoint targeting antibodies.
Keywords: adjuvant; angiogenesis; immunotherapy; metastasis; neoadjuvant.
© 2021 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
RSK is a member of the Scientific Advisory Board of Angiocrine Bioscience Inc., CSTS Healthcare, Novelty Nobility, Nonagen Therapeutics and OncoHost and a consultant to Novelty Nobility, Pharmabcine, and CSTS Healthcare. The other authors declare no conflicts of interest.
Figures
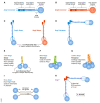
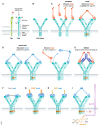

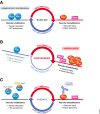

References
-
- Adler AP, Daly C, Parveen AA, Nevins T, Shan J, Fairhurst J, Huang T, Martin J, Papadopoulos N, Yancopoulos GD et al (2014) Abstract 4492: Blockade of angiopoietin‐2 or Tie2 is equally effective at inhibiting tumor growth and reducing tumor vessel density in most human tumor xenograft models. Cancer Res 74: 4492
-
- Al Wadi K, Ghatage P (2016) Efficacy of trebananib (AMG 386) in treating epithelial ovarian cancer. Expert Opin Pharmacother 17: 853–860 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

