Metal Cation-Binding Mechanisms of Q-Proline Peptoid Macrocycles in Solution
- PMID: 34125519
- PMCID: PMC8370751
- DOI: 10.1021/acs.jcim.1c00447
Metal Cation-Binding Mechanisms of Q-Proline Peptoid Macrocycles in Solution
Abstract
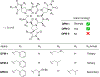
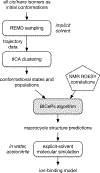
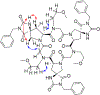
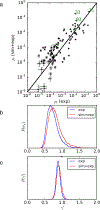
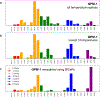
The rational design of foldable and functionalizable peptidomimetic scaffolds requires the concerted application of both computational and experimental methods. Recently, a new class of designed peptoid macrocycle incorporating spiroligomer proline mimics (Q-prolines) has been found to preorganize when bound by monovalent metal cations. To determine the solution-state structure of these cation-bound macrocycles, we employ a Bayesian inference method (BICePs) to reconcile enhanced-sampling molecular simulations with sparse ROESY correlations from experimental NMR studies to predict and design conformational and binding properties of macrocycles as functional scaffolds for peptidomimetics. Conformations predicted to be most populated in solution were then simulated in the presence of explicit cations to yield trajectories with observed binding events, revealing a highly preorganized all-trans amide conformation, whose formation is likely limited by the slow rate of cis/trans isomerization. Interestingly, this conformation differs from a racemic crystal structure solved in the absence of cation. Free energies of cation binding computed from distance-dependent potentials of mean force suggest Na+ has a higher affinity to the macrocycle than K+, with both cations binding much more strongly in acetonitrile than water. The simulated affinities are able to correctly rank the extent to which different macrocycle sequences exhibit preorganization in the presence of different metal cations and solvents, suggesting our approach is suitable for solution-state computational design.
Figures




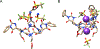
References
-
- Martí-Centelles V; Pandey MD; Burguete MI; Luis SV Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization. 2015. - PubMed
-
- Rajagopalan PT; Benkovic SJ Preorganization and Protein Dynamics in Enzyme Catalysis. Chem. Rec 2002, 2, 24–36. - PubMed
-
- Northrup JD; Wiener JA; Hurley MFD; Hou C-FD; Keller TM; Baxter RHG; Zdilla MJ; Voelz VA; Schafmeister CE Metal-Binding Q-Proline Macrocycles. J. Org. Chem 2021, 86, 4867–4876. - PubMed
-
- Schafmeister CE; Brown ZZ; Gupta S Shape-Programmable Macromolecules. Acc. Chem. Res 2008, 41, 1387–1398. - PubMed
-
- Brown ZZ; Schafmeister CE Synthesis of Hexa–And Pentasubstituted Diketopiperazines From Sterically Hindered Amino Acids. Org. Lett 2010, 12, 1436–1439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

