Circadian Rhythms Within the Female HPG Axis: From Physiology to Etiology
- PMID: 34125877
- PMCID: PMC8256628
- DOI: 10.1210/endocr/bqab117
Circadian Rhythms Within the Female HPG Axis: From Physiology to Etiology
Abstract
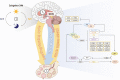
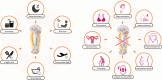
Declining female fertility has become a global health concern. It results partially from an abnormal circadian clock caused by unhealthy diet and sleep habits in modern life. The circadian clock system is a hierarchical network consisting of central and peripheral clocks. It not only controls the sleep-wake and feeding-fasting cycles but also coordinates and maintains the required reproductive activities in the body. Physiologically, the reproductive processes are governed by the hypothalamic-pituitary-gonadal (HPG) axis in a time-dependent manner. The HPG axis releases hormones, generates female characteristics, and achieves fertility. Conversely, an abnormal daily rhythm caused by aberrant clock genes or abnormal environmental stimuli contributes to disorders of the female reproductive system, such as polycystic ovarian syndrome and premature ovarian insufficiency. Therefore, breaking the "time code" of the female reproductive system is crucial. In this paper, we review the interplay between circadian clocks and the female reproductive system and present its regulatory principles, moving from normal physiology regulation to disease etiology.
Keywords: Circadian clock; clock genes; female reproductive disorders; hypothalamic–pituitary–gonadal axis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Lemarchand-Béraud T, Zufferey MM, Reymond M, Rey I. Maturation of the hypothalamo-pituitary-ovarian axis in adolescent girls. J Clin Endocrinol Metab. 1982;54(2):241-246. - PubMed
-
- Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57(4):792-796. - PubMed
-
- Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21(2):67-84. - PubMed
-
- Silva CC, Domínguez R. Clock control of mammalian reproductive cycles: Looking beyond the pre-ovulatory surge of gonadotropins. Rev Endocr Metab Disord. 2020;21(1):149-163. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

