smORFer: a modular algorithm to detect small ORFs in prokaryotes
- PMID: 34125903
- PMCID: PMC8421149
- DOI: 10.1093/nar/gkab477
smORFer: a modular algorithm to detect small ORFs in prokaryotes
Abstract
Emerging evidence places small proteins (≤50 amino acids) more centrally in physiological processes. Yet, their functional identification and the systematic genome annotation of their cognate small open-reading frames (smORFs) remains challenging both experimentally and computationally. Ribosome profiling or Ribo-Seq (that is a deep sequencing of ribosome-protected fragments) enables detecting of actively translated open-reading frames (ORFs) and empirical annotation of coding sequences (CDSs) using the in-register translation pattern that is characteristic for genuinely translating ribosomes. Multiple identifiers of ORFs that use the 3-nt periodicity in Ribo-Seq data sets have been successful in eukaryotic smORF annotation. They have difficulties evaluating prokaryotic genomes due to the unique architecture (e.g. polycistronic messages, overlapping ORFs, leaderless translation, non-canonical initiation etc.). Here, we present a new algorithm, smORFer, which performs with high accuracy in prokaryotic organisms in detecting putative smORFs. The unique feature of smORFer is that it uses an integrated approach and considers structural features of the genetic sequence along with in-frame translation and uses Fourier transform to convert these parameters into a measurable score to faithfully select smORFs. The algorithm is executed in a modular way, and dependent on the data available for a particular organism, different modules can be selected for smORF search.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
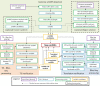

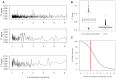


References
-
- Basrai M.A., Hieter P., Boeke J.D.. Small open reading frames: beautiful needles in the haystack. Genome Res. 1997; 7:768–771. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

