Adaptive lymphocyte profile analysis discriminates mild and severe forms of COVID-19 after solid organ transplantation
- PMID: 34126110
- PMCID: PMC8193964
- DOI: 10.1016/j.kint.2021.05.032
Adaptive lymphocyte profile analysis discriminates mild and severe forms of COVID-19 after solid organ transplantation
Abstract
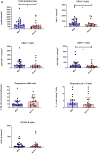
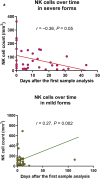
Solid organ transplant recipients are at high risk for the development of severe forms of COVID-19. However, the role of immunosuppression in the morbidity and mortality of the immune phenotype during COVID-19 in transplant recipients remains unknown. In this retrospective study, we compared peripheral blood T and B cell functional and surface markers, as well as serum antibody development during 29 cases of mild (World Health Organization 9-point Ordinal Scale (WOS) of 3-4) and 22 cases of severe COVID-19 (WOS 5-8) in solid organ transplant (72% kidney transplant) recipients hospitalized in our center. Patients who developed severe forms of COVID-19 presented significantly lower CD3+ (median 344/mm3 (inter quartile range 197; 564) vs. 643/mm3 (397; 1251)) and CD8+ T cell counts (124/mm3 (76; 229) vs. 240/mm3 (119; 435)). However, activated CD4+ T cells were significantly more frequent in severe forms (2.9% (1.37; 5.72) vs. 1.4% (0.68; 2.35)), counterbalanced by a significantly higher proportion of Tregs (3.9% (2.35; 5.87) vs. 2.7% (1.9; 3.45)). A marked decrease in the proportion of NK cells was noted only in severe forms. In the B cell compartment, transitional B cells were significantly lower in severe forms (1.2% (0.7; 4.2) vs. 3.6% (2.1; 6.2)). Nonetheless, a majority of transplant recipients developed antibodies against SARS-CoV-2 (77% and 83% in mild and severe forms, respectively). Thus, our data revealed immunological differences between mild and severe forms of COVID-19 in solid organ transplant recipients, similar to previous reports in the immunocompetent population.
Keywords: COVID-19; NK cells; exhaustion; lymphopenia; organ transplantation; regulatory T cells.
Copyright © 2021 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

