Loss of SDHB Promotes Dysregulated Iron Homeostasis, Oxidative Stress, and Sensitivity to Ascorbate
- PMID: 34127497
- PMCID: PMC7616967
- DOI: 10.1158/0008-5472.CAN-20-2936
Loss of SDHB Promotes Dysregulated Iron Homeostasis, Oxidative Stress, and Sensitivity to Ascorbate
Abstract
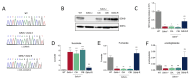
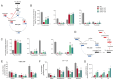
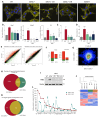
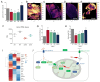
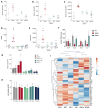
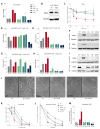
Succinate dehydrogenase is a key enzyme in the tricarboxylic acid cycle and the electron transport chain. All four subunits of succinate dehydrogenase are tumor suppressor genes predisposing to paraganglioma, but only mutations in the SDHB subunit are associated with increased risk of metastasis. Here we generated an Sdhd knockout chromaffin cell line and compared it with Sdhb-deficient cells. Both cell types exhibited similar SDH loss of function, metabolic adaptation, and succinate accumulation. In contrast, Sdhb-/- cells showed hallmarks of mesenchymal transition associated with increased DNA hypermethylation and a stronger pseudo-hypoxic phenotype compared with Sdhd-/- cells. Loss of SDHB specifically led to increased oxidative stress associated with dysregulated iron and copper homeostasis in the absence of NRF2 activation. High-dose ascorbate exacerbated the increase in mitochondrial reactive oxygen species, leading to cell death in Sdhb-/- cells. These data establish a mechanism linking oxidative stress to iron homeostasis that specifically occurs in Sdhb-deficient cells and may promote metastasis. They also highlight high-dose ascorbate as a promising therapeutic strategy for SDHB-related cancers. SIGNIFICANCE: Loss of different succinate dehydrogenase subunits can lead to different cell and tumor phenotypes, linking stronger 2-OG-dependent dioxygenases inhibition, iron overload, and ROS accumulation following SDHB mutation.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–51. - PubMed
-
- Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

