The insulo-opercular cortex encodes food-specific content under controlled and naturalistic conditions
- PMID: 34127675
- PMCID: PMC8203663
- DOI: 10.1038/s41467-021-23885-4
The insulo-opercular cortex encodes food-specific content under controlled and naturalistic conditions
Abstract
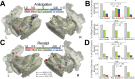
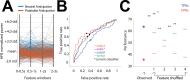
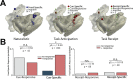
The insulo-opercular network functions critically not only in encoding taste, but also in guiding behavior based on anticipated food availability. However, there remains no direct measurement of insulo-opercular activity when humans anticipate taste. Here, we collect direct, intracranial recordings during a food task that elicits anticipatory and consummatory taste responses, and during ad libitum consumption of meals. While cue-specific high-frequency broadband (70-170 Hz) activity predominant in the left posterior insula is selective for taste-neutral cues, sparse cue-specific regions in the anterior insula are selective for palatable cues. Latency analysis reveals this insular activity is preceded by non-discriminatory activity in the frontal operculum. During ad libitum meal consumption, time-locked high-frequency broadband activity at the time of food intake discriminates food types and is associated with cue-specific activity during the task. These findings reveal spatiotemporally-specific activity in the human insulo-opercular cortex that underlies anticipatory evaluation of food across both controlled and naturalistic settings.
Conflict of interest statement
The authors declare the following competing interests: C.H.H. receives consulting and speaking honoraria from NeuroPace, Boston Scientific, and Ad-Tech. C.J.K. receives salary and equity from Alto Neuroscience. The remaining authors declare no competing interests.
Figures



Similar articles
-
Insulo-opercular cortex generates oroalimentary automatisms in temporal seizures.Epilepsia. 2018 Mar;59(3):583-594. doi: 10.1111/epi.14011. Epub 2018 Feb 2. Epilepsia. 2018. PMID: 29392713
-
Bipolar electro-coagulation with cortextomy in the treatment of insular and insulo-opercular epilepsy explored by stereoelectro-encephalography.Epilepsy Res. 2018 Sep;145:18-26. doi: 10.1016/j.eplepsyres.2018.05.007. Epub 2018 May 24. Epilepsy Res. 2018. PMID: 29803954
-
Visual-gustatory interaction: orbitofrontal and insular cortices mediate the effect of high-calorie visual food cues on taste pleasantness.PLoS One. 2012;7(3):e32434. doi: 10.1371/journal.pone.0032434. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22431974 Free PMC article. Clinical Trial.
-
Functions of the anterior insula in taste, autonomic, and related functions.Brain Cogn. 2016 Dec;110:4-19. doi: 10.1016/j.bandc.2015.07.002. Epub 2015 Aug 12. Brain Cogn. 2016. PMID: 26277487 Review.
-
Brain generators of laser-evoked potentials: from dipoles to functional significance.Neurophysiol Clin. 2003 Dec;33(6):279-92. doi: 10.1016/j.neucli.2003.10.008. Neurophysiol Clin. 2003. PMID: 14678842 Review.
Cited by
-
Naturalistic acute pain states decoded from neural and facial dynamics.bioRxiv [Preprint]. 2024 May 12:2024.05.10.593652. doi: 10.1101/2024.05.10.593652. bioRxiv. 2024. Update in: Nat Commun. 2025 May 11;16(1):4371. doi: 10.1038/s41467-025-59756-5. PMID: 38766098 Free PMC article. Updated. Preprint.
-
Inhibition of POMC neurons in mice undergoing activity-based anorexia selectively blunts food anticipatory activity without affecting body weight or food intake.Am J Physiol Regul Integr Comp Physiol. 2022 Mar 1;322(3):R219-R227. doi: 10.1152/ajpregu.00313.2021. Epub 2022 Jan 19. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 35043681 Free PMC article.
-
An orexigenic subnetwork within the human hippocampus.Nature. 2023 Sep;621(7978):381-388. doi: 10.1038/s41586-023-06459-w. Epub 2023 Aug 30. Nature. 2023. PMID: 37648849 Free PMC article.
-
Naturalistic acute pain states decoded from neural and facial dynamics.Nat Commun. 2025 May 11;16(1):4371. doi: 10.1038/s41467-025-59756-5. Nat Commun. 2025. PMID: 40350488 Free PMC article.
-
Increased functional connectivity following ingestion of dried bonito soup.Front Nutr. 2024 Apr 3;11:1354245. doi: 10.3389/fnut.2024.1354245. eCollection 2024. Front Nutr. 2024. PMID: 38633605 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

