SARS-CoV-2 infection and transmission in the North American deer mouse
- PMID: 34127676
- PMCID: PMC8203675
- DOI: 10.1038/s41467-021-23848-9
SARS-CoV-2 infection and transmission in the North American deer mouse
Abstract
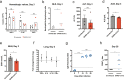
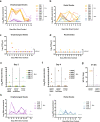
Widespread circulation of SARS-CoV-2 in humans raises the theoretical risk of reverse zoonosis events with wildlife, reintroductions of SARS-CoV-2 into permissive nondomesticated animals. Here we report that North American deer mice (Peromyscus maniculatus) are susceptible to SARS-CoV-2 infection following intranasal exposure to a human isolate, resulting in viral replication in the upper and lower respiratory tract with little or no signs of disease. Further, shed infectious virus is detectable in nasal washes, oropharyngeal and rectal swabs, and viral RNA is detectable in feces and occasionally urine. We further show that deer mice are capable of transmitting SARS-CoV-2 to naïve deer mice through direct contact. The extent to which these observations may translate to wild deer mouse populations remains unclear, and the risk of reverse zoonosis and/or the potential for the establishment of Peromyscus rodents as a North American reservoir for SARS-CoV-2 remains unknown.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

