Vaccination with BCGΔBCG1419c protects against pulmonary and extrapulmonary TB and is safer than BCG
- PMID: 34127755
- PMCID: PMC8203684
- DOI: 10.1038/s41598-021-91993-8
Vaccination with BCGΔBCG1419c protects against pulmonary and extrapulmonary TB and is safer than BCG
Abstract
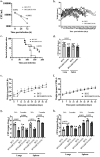
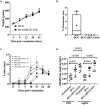
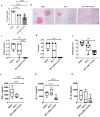
A single intradermal vaccination with an antibiotic-less version of BCGΔBCG1419c given to guinea pigs conferred a significant improvement in outcome following a low dose aerosol exposure to M. tuberculosis compared to that provided by a single dose of BCG Pasteur. BCGΔBCG1419c was more attenuated than BCG in murine macrophages, athymic, BALB/c, and C57BL/6 mice. In guinea pigs, BCGΔBCG1419c was at least as attenuated as BCG and induced similar dermal reactivity to that of BCG. Vaccination of guinea pigs with BCGΔBCG1419c resulted in increased anti-PPD IgG compared with those receiving BCG. Guinea pigs vaccinated with BCGΔBCG1419c showed a significant reduction of M. tuberculosis replication in lungs and spleens compared with BCG, as well as a significant reduction of pulmonary and extrapulmonary tuberculosis (TB) pathology measured using pathology scores recorded at necropsy. Evaluation of cytokines produced in lungs of infected guinea pigs showed that BCGΔBCG1419c significantly reduced TNF-α and IL-17 compared with BCG-vaccinated animals, with no changes in IL-10. This work demonstrates a significantly improved protection against pulmonary and extrapulmonary TB provided by BCGΔBCG1419c in susceptible guinea pigs together with an increased safety compared with BCG in several models. These results support the continued development of BCGΔBCG1419c as an effective vaccine for TB.
Conflict of interest statement
M.J.A.S., M.A.F.V. and C.P.R. are co-inventors on a patent on BCGΔBCG1419c held by the Centro de Investigación y Asistencia en Tecnología y diseño del Estado de Jalisco (CIATEJ), A.C. and Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”.
Figures


References
-
- World Health Organization. Global Tuberculosis Report 2020.https://www.who.int/publications/i/item/9789240013131(2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

