Hypertension-induced cognitive impairment: from pathophysiology to public health
- PMID: 34127835
- PMCID: PMC8202227
- DOI: 10.1038/s41581-021-00430-6
Hypertension-induced cognitive impairment: from pathophysiology to public health
Abstract
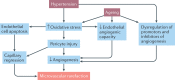
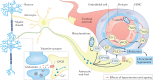
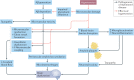
Hypertension affects two-thirds of people aged >60 years and significantly increases the risk of both vascular cognitive impairment and Alzheimer's disease. Hypertension compromises the structural and functional integrity of the cerebral microcirculation, promoting microvascular rarefaction, cerebromicrovascular endothelial dysfunction and neurovascular uncoupling, which impair cerebral blood supply. In addition, hypertension disrupts the blood-brain barrier, promoting neuroinflammation and exacerbation of amyloid pathologies. Ageing is characterized by multifaceted homeostatic dysfunction and impaired cellular stress resilience, which exacerbate the deleterious cerebromicrovascular effects of hypertension. Neuroradiological markers of hypertension-induced cerebral small vessel disease include white matter hyperintensities, lacunar infarcts and microhaemorrhages, all of which are associated with cognitive decline. Use of pharmaceutical and lifestyle interventions that reduce blood pressure, in combination with treatments that promote microvascular health, have the potential to prevent or delay the pathogenesis of vascular cognitive impairment and Alzheimer's disease in patients with hypertension.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

