A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study
- PMID: 34127959
- PMCID: PMC8189543
- DOI: 10.1016/j.eclinm.2021.100941
A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study
Abstract
Background: Effective home treatment algorithms implemented based on a pathophysiologic and pharmacologic rationale to accelerate recovery and prevent hospitalisation of patients with early coronavirus disease 2019 (COVID-19) would have major implications for patients and health system.
Methods: This academic, matched-cohort study compared outcomes of 90 consecutive consenting patients with mild COVID-19 treated at home by their family physicians between October 2020 and January 2021 in Northern and Central Italy, according to the proposed recommendation algorithm, with outcomes for 90 age-, sex-, and comorbidities-matched patients who received other therapeutic regimens. Primary outcome was time to resolution of major symptoms. Secondary outcomes included prevention of hospitalisation. Analyses were by intention-to-treat.
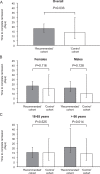
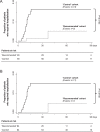
Findings: All patients achieved complete remission. The median [IQR] time to resolution of major symptoms was 18 [14-23] days in the 'recommended schedule' cohort and 14 [7-30] days in the matched 'control' cohort (p = 0·033). Other symptoms persisted in a lower percentage of patients in the 'recommended' than in the 'control' cohort (23·3% versus 73·3%, respectively, p<0·0001) and for a shorter period (p = 0·0107). Two patients in the 'recommended' cohort were hospitalised compared to 13 (14·4%) controls (p = 0·0103). The prevention algorithm reduced the days and cumulative costs of hospitalisation by >90%.
Interpretation: Implementation of an early home treatment algorithm failed to accelerate recovery from major symptoms of COVID-19, but reduced the risk of hospitalisation and related treatment costs. Given the study design, additional research would be required to consolidate the proposed treatment recommendations.
Funding: Fondazione Cav.Lav. Carlo Pesenti.
Keywords: COVID-19; Early symptoms at home; Family physicians; Matched-cohort observational study; SARS-CoV-2; Simple home-therapy algorithm.
© 2021 The Authors.
Conflict of interest statement
We declare that we have no conflicts of interest.
Figures

Comment in
-
Further data on use of NSAIDs for the home-care therapy of COVID-19.Intern Emerg Med. 2023 Aug;18(5):1599-1602. doi: 10.1007/s11739-023-03272-1. Epub 2023 Apr 12. Intern Emerg Med. 2023. PMID: 37046061 Free PMC article. No abstract available.
References
-
- Johns Hopkins C.S.S.E. COVID-19 Map - Johns Hopkins coronavirus resource center. Accessed March 19, 2021. Available at: https://www.coronavirus.jhu.edu.
-
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a Review. JAMA. 2020;323:1824–1836. - PubMed
-
- Kupferschmidt K., Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367:1412–1413. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

