This is a preprint.
Evolution of enhanced innate immune evasion by the SARS-CoV-2 B.1.1.7 UK variant
- PMID: 34127972
- PMCID: PMC8202424
- DOI: 10.1101/2021.06.06.446826
Evolution of enhanced innate immune evasion by the SARS-CoV-2 B.1.1.7 UK variant
Update in
-
Evolution of enhanced innate immune evasion by SARS-CoV-2.Nature. 2022 Feb;602(7897):487-495. doi: 10.1038/s41586-021-04352-y. Epub 2021 Dec 23. Nature. 2022. PMID: 34942634 Free PMC article.
Abstract
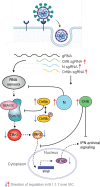
Emergence of SARS-CoV-2 variants, including the globally successful B.1.1.7 lineage, suggests viral adaptations to host selective pressures resulting in more efficient transmission. Although much effort has focused on Spike adaptation for viral entry and adaptive immune escape, B.1.1.7 mutations outside Spike likely contribute to enhance transmission. Here we used unbiased abundance proteomics, phosphoproteomics, mRNA sequencing and viral replication assays to show that B.1.1.7 isolates more effectively suppress host innate immune responses in airway epithelial cells. We found that B.1.1.7 isolates have dramatically increased subgenomic RNA and protein levels of Orf9b and Orf6, both known innate immune antagonists. Expression of Orf9b alone suppressed the innate immune response through interaction with TOM70, a mitochondrial protein required for RNA sensing adaptor MAVS activation, and Orf9b binding and activity was regulated via phosphorylation. We conclude that B.1.1.7 has evolved beyond the Spike coding region to more effectively antagonise host innate immune responses through upregulation of specific subgenomic RNA synthesis and increased protein expression of key innate immune antagonists. We propose that more effective innate immune antagonism increases the likelihood of successful B.1.1.7 transmission, and may increase in vivo replication and duration of infection.
Figures




References
-
- Volz E. et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 593, 266–269 (2021). - PubMed
Publication types
Grants and funding
- U54 CA260560/CA/NCI NIH HHS/United States
- 207498/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- HHSN272201400008C/AI/NIAID NIH HHS/United States
- R01 AI120694/AI/NIAID NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- U19 AI135990/AI/NIAID NIH HHS/United States
- F32 CA239333/CA/NCI NIH HHS/United States
- R01 GM133981/GM/NIGMS NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
- P50 AI150476/AI/NIAID NIH HHS/United States
- R01 AI143292/AI/NIAID NIH HHS/United States
- U19 AI135972/AI/NIAID NIH HHS/United States
- 75N93021C00014/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
