Inhibitory control in neuronal networks relies on the extracellular matrix integrity
- PMID: 34128077
- PMCID: PMC8257544
- DOI: 10.1007/s00018-021-03861-3
Inhibitory control in neuronal networks relies on the extracellular matrix integrity
Abstract
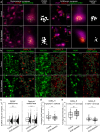
Inhibitory control is essential for the regulation of neuronal network activity, where excitatory and inhibitory synapses can act synergistically, reciprocally, and antagonistically. Sustained excitation-inhibition (E-I) balance, therefore, relies on the orchestrated adjustment of excitatory and inhibitory synaptic strength. While growing evidence indicates that the brain's extracellular matrix (ECM) is a crucial regulator of excitatory synapse plasticity, it remains unclear whether and how the ECM contributes to inhibitory control in neuronal networks. Here we studied the simultaneous changes in excitatory and inhibitory connectivity after ECM depletion. We demonstrate that the ECM supports the maintenance of E-I balance by retaining inhibitory connectivity. Quantification of synapses and super-resolution microscopy showed that depletion of the ECM in mature neuronal networks preferentially decreases the density of inhibitory synapses and the size of individual inhibitory postsynaptic scaffolds. The reduction of inhibitory synapse density is partially compensated by the homeostatically increasing synaptic strength via the reduction of presynaptic GABAB receptors, as indicated by patch-clamp measurements and GABAB receptor expression quantifications. However, both spiking and bursting activity in neuronal networks is increased after ECM depletion, as indicated by multi-electrode recordings. With computational modelling, we determined that ECM depletion reduces the inhibitory connectivity to an extent that the inhibitory synapse scaling does not fully compensate for the reduced inhibitory synapse density. Our results indicate that the brain's ECM preserves the balanced state of neuronal networks by supporting inhibitory control via inhibitory synapse stabilization, which expands the current understanding of brain activity regulation.
Keywords: E-I balance; ECM; Electrophysiology; Inhibitory synapse; Neuronal network activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y. Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci. 2014;34:6164–6176. doi: 10.1523/JNEUROSCI.3458-13.2014. - DOI - PMC - PubMed
-
- Beggs JM, Klukas J, Chen W. Connectivity and Dynamics in Local Cortical Networks. In: Jirsa VK, McIntosh AR, editors. Handbook of Brain Connectivity. Berlin: Springer; 2007. pp. 91–116.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

