Structure of Tau filaments in Prion protein amyloidoses
- PMID: 34128081
- PMCID: PMC8270882
- DOI: 10.1007/s00401-021-02336-w
Structure of Tau filaments in Prion protein amyloidoses
Abstract
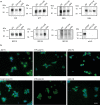
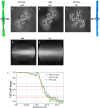
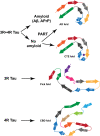
In human neurodegenerative diseases associated with the intracellular aggregation of Tau protein, the ordered cores of Tau filaments adopt distinct folds. Here, we analyze Tau filaments isolated from the brain of individuals affected by Prion-Protein cerebral amyloid angiopathy (PrP-CAA) with a nonsense mutation in the PRNP gene that leads to early termination of translation of PrP (Q160Ter or Q160X), and Gerstmann-Sträussler-Scheinker (GSS) disease, with a missense mutation in the PRNP gene that leads to an amino acid substitution at residue 198 (F198S) of PrP. The clinical and neuropathologic phenotypes associated with these two mutations in PRNP are different; however, the neuropathologic analyses of these two genetic variants have consistently shown the presence of numerous neurofibrillary tangles (NFTs) made of filamentous Tau aggregates in neurons. We report that Tau filaments in PrP-CAA (Q160X) and GSS (F198S) are composed of 3-repeat and 4-repeat Tau isoforms, having a striking similarity to NFTs in Alzheimer disease (AD). In PrP-CAA (Q160X), Tau filaments are made of both paired helical filaments (PHFs) and straight filaments (SFs), while in GSS (F198S), only PHFs were found. Mass spectrometry analyses of Tau filaments extracted from PrP-CAA (Q160X) and GSS (F198S) brains show the presence of post-translational modifications that are comparable to those seen in Tau aggregates from AD. Cryo-EM analysis reveals that the atomic models of the Tau filaments obtained from PrP-CAA (Q160X) and GSS (F198S) are identical to those of the Tau filaments from AD, and are therefore distinct from those of Pick disease, chronic traumatic encephalopathy, and corticobasal degeneration. Our data support the hypothesis that in the presence of extracellular amyloid deposits and regardless of the primary amino acid sequence of the amyloid protein, similar molecular mechanisms are at play in the formation of identical Tau filaments.
Keywords: APrP; Cryo-EM; GSS; Neurodegeneration; PrP-CAA; Tau.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Cryo-EM structures of prion protein filaments from Gerstmann-Sträussler-Scheinker disease.Acta Neuropathol. 2022 Sep;144(3):509-520. doi: 10.1007/s00401-022-02461-0. Epub 2022 Jul 12. Acta Neuropathol. 2022. PMID: 35819518 Free PMC article.
-
Gerstmann-Sträussler-Scheinker Disease with F198S Mutation Induces Independent Tau and Prion Protein Pathologies in Bank Voles.Biomolecules. 2022 Oct 21;12(10):1537. doi: 10.3390/biom12101537. Biomolecules. 2022. PMID: 36291746 Free PMC article.
-
Detection of tau in Gerstmann-Sträussler-Scheinker disease (PRNP F198S) by [18F]Flortaucipir PET.Acta Neuropathol Commun. 2018 Oct 29;6(1):114. doi: 10.1186/s40478-018-0608-z. Acta Neuropathol Commun. 2018. PMID: 30373672 Free PMC article.
-
Hereditary prion protein amyloidoses.Clin Lab Med. 2003 Mar;23(1):65-85, viii. doi: 10.1016/s0272-2712(02)00064-1. Clin Lab Med. 2003. PMID: 12733425 Review.
-
How an Infection of Sheep Revealed Prion Mechanisms in Alzheimer's Disease and Other Neurodegenerative Disorders.Int J Mol Sci. 2021 May 4;22(9):4861. doi: 10.3390/ijms22094861. Int J Mol Sci. 2021. PMID: 34064393 Free PMC article. Review.
Cited by
-
Prion-like strain effects in tauopathies.Cell Tissue Res. 2023 Apr;392(1):179-199. doi: 10.1007/s00441-022-03620-1. Epub 2022 Apr 23. Cell Tissue Res. 2023. PMID: 35460367 Free PMC article. Review.
-
Hierarchical organization and assembly of the archaeal cell sheath from an amyloid-like protein.Nat Commun. 2023 Oct 23;14(1):6720. doi: 10.1038/s41467-023-42368-2. Nat Commun. 2023. PMID: 37872154 Free PMC article.
-
Tau and neurodegeneration.Cytoskeleton (Hoboken). 2024 Jan;81(1):95-102. doi: 10.1002/cm.21812. Epub 2023 Dec 10. Cytoskeleton (Hoboken). 2024. PMID: 38073060 Free PMC article. Review.
-
Novel insights into D-Pinitol based therapies: a link between tau hyperphosphorylation and insulin resistance.Neural Regen Res. 2024 Feb;19(2):289-295. doi: 10.4103/1673-5374.379015. Neural Regen Res. 2024. PMID: 37488880 Free PMC article. Review.
-
Ligands for Protein Fibrils of Amyloid-β, α-Synuclein, and Tau.Chem Rev. 2025 Jun 11;125(11):5282-5348. doi: 10.1021/acs.chemrev.4c00838. Epub 2025 May 6. Chem Rev. 2025. PMID: 40327808 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

