In vivo evaluation of temperature-responsive antimicrobial-loaded PNIPAAm hydrogels for prevention of surgical site infection
- PMID: 34128323
- PMCID: PMC8608705
- DOI: 10.1002/jbm.b.34894
In vivo evaluation of temperature-responsive antimicrobial-loaded PNIPAAm hydrogels for prevention of surgical site infection
Abstract
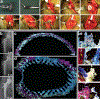
Surgical site infections (SSIs) are a persistent clinical challenge. Local antimicrobial delivery may reduce the risk of SSI by increasing drug concentrations and distribution in vulnerable surgical sites compared to what is achieved using systemic antimicrobial prophylaxis alone. In this work, we describe a comprehensive in vivo evaluation of the safety and efficacy of poly(N-isopropylacrylamide-co-dimethylbutyrolactone acrylamide-co-Jeffamine M-1000 acrylamide) [PNDJ], an injectable temperature-responsive hydrogel carrier for antimicrobial delivery in surgical sites. Biodistribution data indicate that PNDJ is primarily cleared via the liver and kidneys following drug delivery. Antimicrobial-loaded PNDJ was generally well-tolerated locally and systemically when applied in bone, muscle, articulating joints, and intraperitoneal space, although mild renal toxicity consistent with the released antimicrobials was identified at high doses in rats. Dosing of PNDJ at bone-implant interfaces did not affect normal tissue healing and function of orthopedic implants in a transcortical plug model in rabbits and in canine total hip arthroplasty. Finally, PNDJ was effective at preventing recurrence of implant-associated MSSA and MRSA osteomyelitis in rabbits, showing a trend toward outperforming commercially available antimicrobial-loaded bone cement and systemic antimicrobial administration. These studies indicate that antimicrobial-loaded PNDJ hydrogels are well-tolerated and could reduce incidence of SSI in a variety of surgical procedures.
Keywords: NIPAAm polymers; hydrogel biocompatibility; local antimicrobial delivery; surgical site infection; sustained release.
© 2021 Wiley Periodicals LLC.
Figures



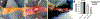
References
-
- Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, Tsai TC, Ko CY, Bilimoria KY. Underlying Reasons Associated With Hospital Readmission Following Surgery in the United States. JAMA. 2015;313:483. - PubMed
-
- Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The Economic Impact of Periprosthetic Infections Following Total Knee Arthroplasty at a Specialized Tertiary-Care Center. J Arthroplasty. 2014;29:929–32. - PubMed
-
- de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

