European guideline on indications, performance and clinical impact of 13 C-breath tests in adult and pediatric patients: An EAGEN, ESNM, and ESPGHAN consensus, supported by EPC
- PMID: 34128346
- PMCID: PMC8259225
- DOI: 10.1002/ueg2.12099
European guideline on indications, performance and clinical impact of 13 C-breath tests in adult and pediatric patients: An EAGEN, ESNM, and ESPGHAN consensus, supported by EPC
Abstract
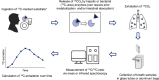
Introduction: 13 C-breath tests are valuable, noninvasive diagnostic tests that can be widely applied for the assessment of gastroenterological symptoms and diseases. Currently, the potential of these tests is compromised by a lack of standardization regarding performance and interpretation among expert centers.
Methods: This consensus-based clinical practice guideline defines the clinical indications, performance, and interpretation of 13 C-breath tests in adult and pediatric patients. A balance between scientific evidence and clinical experience was achieved by a Delphi consensus that involved 43 experts from 18 European countries. Consensus on individual statements and recommendations was established if ≥ 80% of reviewers agreed and <10% disagreed.
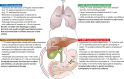
Results: The guideline gives an overview over general methodology of 13 C-breath testing and provides recommendations for the use of 13 C-breath tests to diagnose Helicobacter pylori infection, measure gastric emptying time, and monitor pancreatic exocrine and liver function in adult and pediatric patients. Other potential applications of 13 C-breath testing are summarized briefly. The recommendations specifically detail when and how individual 13 C-breath tests should be performed including examples for well-established test protocols, patient preparation, and reporting of test results.
Conclusion: This clinical practice guideline should improve pan-European harmonization of diagnostic approaches to symptoms and disorders, which are very common in specialist and primary care gastroenterology practice, both in adult and pediatric patients. In addition, this guideline identifies areas of future clinical research involving the use of 13 C-breath tests.
Keywords: breathtest; diagnosis; gastroenterology; gastroparesis; helicobacter pylori; liver cirrhosis; motility; pancreatic exocrine insufficiency; pancreatitis.
© 2021 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Oliver Goetze received financial support/honoraria for clinical studies/lectures from Kibion, Mayoly Spindler Laboratories. Stephan L. Haas received honoraria by Mylan for oral presentations. The other authors have nothing to disclose.
Figures
Similar articles
-
European guideline on indications, performance, and clinical impact of hydrogen and methane breath tests in adult and pediatric patients: European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Neurogastroenterology and Motility, and European Society for Paediatric Gastroenterology Hepatology and Nutrition consensus.United European Gastroenterol J. 2022 Feb;10(1):15-40. doi: 10.1002/ueg2.12133. Epub 2021 Aug 25. United European Gastroenterol J. 2022. PMID: 34431620 Free PMC article. Review.
-
13C-breath tests: current state of the art and future directions.Dig Liver Dis. 2007 Sep;39(9):795-805. doi: 10.1016/j.dld.2007.06.012. Epub 2007 Jul 25. Dig Liver Dis. 2007. PMID: 17652042 Review.
-
An ESPGHAN Position Paper on the Use of Breath Testing in Paediatric Gastroenterology.J Pediatr Gastroenterol Nutr. 2022 Jan 1;74(1):123-137. doi: 10.1097/MPG.0000000000003245. J Pediatr Gastroenterol Nutr. 2022. PMID: 34292218
-
[Importance of functional diagnostics in gastroenterology].Internist (Berl). 2018 Jan;59(1):25-37. doi: 10.1007/s00108-017-0359-0. Internist (Berl). 2018. PMID: 29230485 Review. German.
-
[Breath tests in the diagnosis of gastrointestinal diseases].Gastroenterol Hepatol. 2005 Aug-Sep;28(7):407-16. doi: 10.1157/13077762. Gastroenterol Hepatol. 2005. PMID: 16137476 Review. Spanish.
Cited by
-
The [13 C]octanoic acid breath test for gastric emptying quantification: A focus on nutrition and modeling.Lipids. 2022 Jul;57(4-5):205-219. doi: 10.1002/lipd.12352. Epub 2022 Jul 7. Lipids. 2022. PMID: 35799422 Free PMC article. Review.
-
AGA-PancreasFest Joint Symposium on Exocrine Pancreatic Insufficiency.Gastro Hep Adv. 2022 Nov 15;2(3):395-411. doi: 10.1016/j.gastha.2022.11.008. eCollection 2023. Gastro Hep Adv. 2022. PMID: 39132652 Free PMC article. Review.
-
UEG journal's editorial team.United European Gastroenterol J. 2022 Dec;10(10):1041-1043. doi: 10.1002/ueg2.12340. Epub 2022 Nov 24. United European Gastroenterol J. 2022. PMID: 36424368 Free PMC article. No abstract available.
-
Helicobacter pylori Infections in Children.Antibiotics (Basel). 2023 Sep 12;12(9):1440. doi: 10.3390/antibiotics12091440. Antibiotics (Basel). 2023. PMID: 37760736 Free PMC article. Review.
-
European Consensus on Malabsorption-UEG & SIGE, LGA, SPG, SRGH, CGS, ESPCG, EAGEN, ESPEN, and ESPGHAN. Part 1: Definitions, Clinical Phenotypes, and Diagnostic Testing for Malabsorption.United European Gastroenterol J. 2025 May;13(4):599-613. doi: 10.1002/ueg2.70012. Epub 2025 Mar 25. United European Gastroenterol J. 2025. PMID: 40129317 Free PMC article.
References
-
- Sonyi M, Keller J, Fox M, Hammer HF. Development of a multinational clinical practice guideline: a practical structured procedure. Basel: Digestive diseases; 2020. - PubMed
-
- Keller J, Franke A, Storr M, Wiedbrauck F, Schirra J. Clinically relevant breath tests in gastroenterological diagnostics‐‐recommendations of the German Society for Neurogastroenterology and Motility as well as the German Society for Digestive and Metabolic Diseases. Z Gastroenterol. 2005;43 (9):1071–90. - PubMed
-
- Schoeller DA, Schneider JF, Solomons NW, Watkins JB, Klein PD. Clinical diagnosis with the stable isotope 13C in CO2 breath tests: methodology and fundamental considerations. J Lab Clin Med. 1977;90 (3):412–21. - PubMed
-
- Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection‐the Maastricht V/Florence consensus report. Gut. 2017;66 (1):6–30. - PubMed
-
- Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol Nutr. 2017;64 (6):991–1003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

